(a) Electron diffraction experiments reveal that the distance between the centers of the carbon (C) and oxygen (O) atoms in a carbon monoxide molecule is about 1.100 x 10-10 m. Where is the center of mass of a CO molecule? (b) In an ammonia molecule (NH3), the atoms are arranged in a pyramid.* The three hydrogen atoms (H) form an equilateral triangle at the base of a pyramid. The distance between the centers of the hydrogen atoms is 1.628 x 10-10 m; thus, the center of the triangle is 9.399 x 10-11 m from each hydrogen atom. The nitrogen atom (N) sits at the apex of the pyramid. The distance between the center of the nitrogen atom and any hydrogen atom is 1.014 x 10-10 m. Where is the center of mass of the ammonia molecule?
(a) Electron diffraction experiments reveal that the distance between the centers of the carbon (C) and oxygen (O) atoms in a carbon monoxide molecule is about 1.100 x 10-10 m. Where is the center of mass of a CO molecule? (b) In an ammonia molecule (NH3), the atoms are arranged in a pyramid.* The three hydrogen atoms (H) form an equilateral triangle at the base of a pyramid. The distance between the centers of the hydrogen atoms is 1.628 x 10-10 m; thus, the center of the triangle is 9.399 x 10-11 m from each hydrogen atom. The nitrogen atom (N) sits at the apex of the pyramid. The distance between the center of the nitrogen atom and any hydrogen atom is 1.014 x 10-10 m. Where is the center of mass of the ammonia molecule?
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter36: Applications Of The Wave Model
Section: Chapter Questions
Problem 64PQ
Related questions
Question
100%
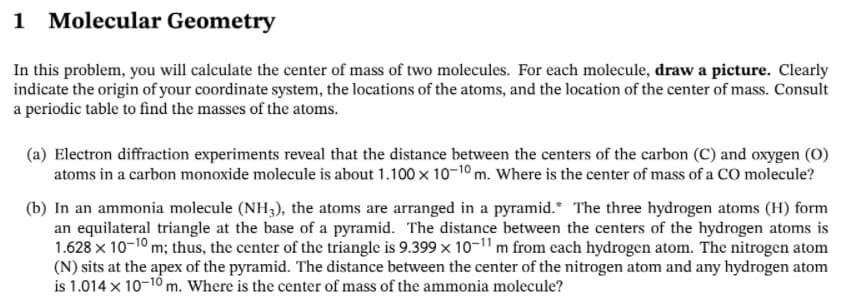
Transcribed Image Text:1 Molecular Geometry
In this problem, you will calculate the center of mass of two molecules. For each molecule, draw a picture. Clearly
indicate the origin of your coordinate system, the locations of the atoms, and the location of the center of mass. Consult
a periodic table to find the masses of the atoms.
(a) Electron diffraction experiments reveal that the distance between the centers of the carbon (C) and oxygen (O)
atoms in a carbon monoxide molecule is about 1.100 x 10-10 m. Where is the center of mass of a CO molecule?
(b) In an ammonia molecule (NH3), the atoms are arranged in a pyramid.* The three hydrogen atoms (H) form
an equilateral triangle at the base of a pyramid. The distance between the centers of the hydrogen atoms is
1.628 x 10-10 m; thus, the center of the triangle is 9.399 x 10-1" m from cach hydrogen atom. The nitrogen atom
(N) sits at the apex of the pyramid. The distance between the center of the nitrogen atom and any hydrogen atom
is 1.014 x 10-10 m. Where is the center of mass of the ammonia molecule?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning