(a) Explain why fluorescence spectroscopy gives a more accurate determination of vitamin B1 than absorption spectroscopy. (b) Provide an annotated sketch of the excitation/emission spectrum as well as an annotated energy profile diagram of the vitamin B1 derivative, thiochrome.
(a) Explain why fluorescence spectroscopy gives a more accurate determination of vitamin B1 than absorption spectroscopy. (b) Provide an annotated sketch of the excitation/emission spectrum as well as an annotated energy profile diagram of the vitamin B1 derivative, thiochrome.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter19: The Tricarboxylic Acid Cycle
Section: Chapter Questions
Problem 5P: Understanding the Action of Fluoroacetate on the TCA Cycle In a tissue where the TCA cycle has been...
Related questions
Question
Transcribed Image Text:Part 1
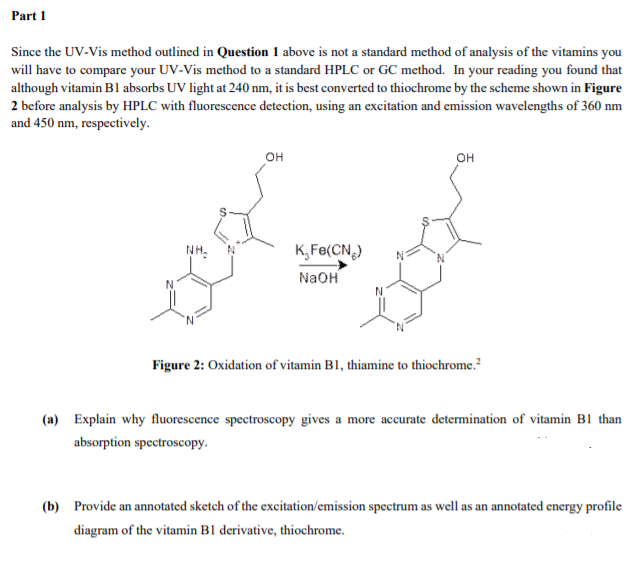
Since the UV-Vis method outlined in Question 1 above is not a standard method of analysis of the vitamins you
will have to compare your UV-Vis method to a standard HPLC or GC method. In your reading you found that
although vitamin B1 absorbs UV light at 240 nm, it is best converted to thiochrome by the scheme shown in Figure
2 before analysis by HPLC with fluorescence detection, using an excitation and emission wavelengths of 360 nm
and 450 nm, respectively.
он
он
NH:
K, Fe(CN,)
NaOH
Figure 2: Oxidation of vitamin B1, thiamine to thiochrome.?
(a) Explain why fluorescence spectroscopy gives a more accurate determination of vitamin B1 than
absorption spectroscopy.
(b) Provide an annotated sketch of the excitation/emission spectrum as well as an annotated energy profile
diagram of the vitamin B1 derivative, thiochrome.
Transcribed Image Text:Part 2
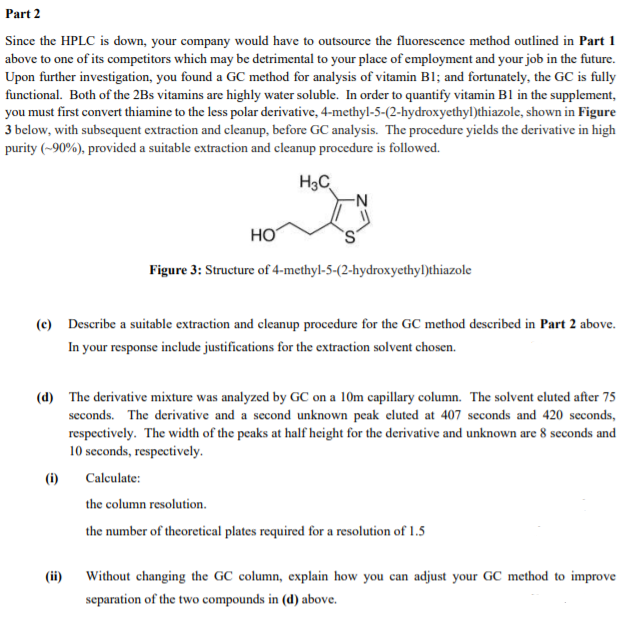
Since the HPLC is down, your company would have to outsource the fluorescence method outlined in Part 1
above to one of its competitors which may be detrimental to your place of employment and your job in the future.
Upon further investigation, you found a GC method for analysis of vitamin B1; and fortunately, the GC is fully
functional. Both of the 2Bs vitamins are highly water soluble. In order to quantify vitamin B1 in the supplement,
you must first convert thiamine to the less polar derivative, 4-methyl-5-(2-hydroxyethyl)thiazole, shown in Figure
3 below, with subsequent extraction and cleanup, before GC analysis. The procedure yields the derivative in high
purity (~90%), provided a suitable extraction and cleanup procedure is followed.
H3C
HO
Figure 3: Structure of 4-methyl-5-(2-hydroxyethyl)thiazole
(c) Describe a suitable extraction and cleanup procedure for the GC method described in Part 2 above.
In your response include justifications for the extraction solvent chosen.
(d) The derivative mixture was analyzed by GC on a 10m capillary column. The solvent eluted after 75
seconds. The derivative and a second unknown peak eluted at 407 seconds and 420 seconds,
respectively. The width of the peaks at half height for the derivative and unknown are 8 seconds and
10 seconds, respectively.
(i)
Calculate:
the column resolution.
the number of theoretical plates required for a resolution of 1.5
(ii)
Without changing the GC column, explain how you can adjust your GC method to improve
separation of the two compounds in (d) above.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax
