(a) Fe + Mn2+ Edit | Incorrect. Is your reaction balanced? (b) Cr + Pb2+ 2+ Cr+ Pb 3+ - 2Cr +3Pb Edit ] Incorrect. Is Fe the stronger reducing agent? (c) Ag* + Fe - Edit X Incorrect. Is your reaction balanced? Is Ag the stronger reducing agent? (d) Ag + Au3+ →
(a) Fe + Mn2+ Edit | Incorrect. Is your reaction balanced? (b) Cr + Pb2+ 2+ Cr+ Pb 3+ - 2Cr +3Pb Edit ] Incorrect. Is Fe the stronger reducing agent? (c) Ag* + Fe - Edit X Incorrect. Is your reaction balanced? Is Ag the stronger reducing agent? (d) Ag + Au3+ →
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter19: Redox Reactions
Section: Chapter Questions
Problem 67A
Related questions
Question
Use the table below to predict the outcome of the following reactions. Note: If no reaction occurs, enter unbalanced left part of the equation and then click "NR" on your ChemPad palette. Do not use the keyboard to enter physical states and "NR". If a reaction occurs, enter a balanced chemical equation for it.
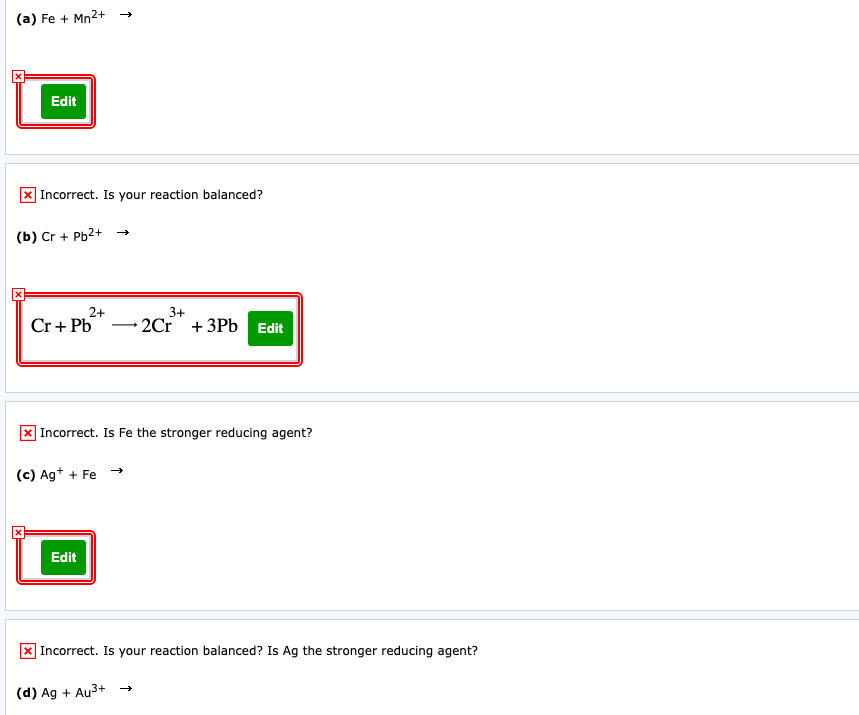
Transcribed Image Text:(a) Fe + Mn2+
Edit
x Incorrect. Is your reaction balanced?
(b) Cr + Pb2+ →
2+
-2Cr + 3Pb Edit
3+
Cr+ Pb
X Incorrect. Is Fe the stronger reducing agent?
(c) Ag+ + Fe
Edit
X Incorrect. Is your reaction balanced? Is Ag the stronger reducing agent?
(d) Ag + Au3+ →
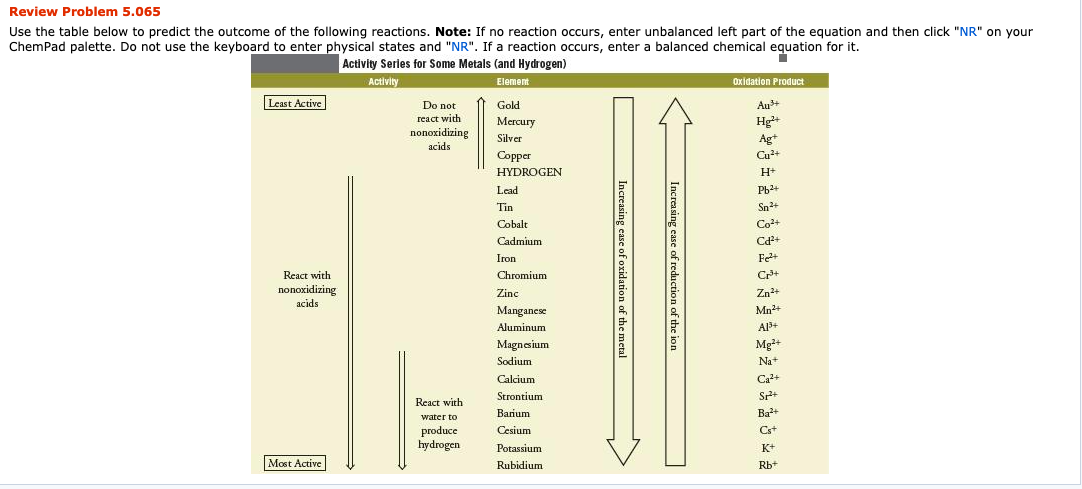
Transcribed Image Text:Review Problem 5.065
Use the table below to predict the outcome of the following reactions. Note: If no reaction occurs, enter unbalanced left part of the equation and then click "NR" on your
ChemPad palette. Do not use the keyboard to enter physical states and "NR". If a reaction occurs, enter a balanced chemical equation for it.
Activity Series for Some Metals (and Hydrogen)
Activity
Oxidation Product
Element
Least Active
Do not
Au*+
Gold
react with
Mercury
Hg+
nonoxidizing
Silver
Ag+
Cu2+
аcids
Copper
HYDROGEN
H+
Lead
Pb2+
Tin
Sn+
Cobalt
Co2+
Cadmium
Cd+
Iron
Fet
React with.
Chromium
Cr+
nonoxidizing
Zinc
Zn2+
acids
Manganese
Mn2+
Aluminum
Al+
Magnesium
Mg*+
Sodium
Na+
Calcium
Ca2+
Strontium
Sr+
React with
Barium
Ba+
water to
produce
hydrogen
Cesium
Cst
Potassium
K+
Most Active
Rubidium
Rb+
Increasing ease of reduction of the ion
Increasing ease of oxidation of the metal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
