A galvanic cell is powered by the following redox reaction: 5 Cl,(9) + I,(s) + 6 H,O(1) - 10 Cl (aq) + 2 IO,(aq) + 12 H"(aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. O-0 Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E° = Ov Round your answer to 2 decimal places.
A galvanic cell is powered by the following redox reaction: 5 Cl,(9) + I,(s) + 6 H,O(1) - 10 Cl (aq) + 2 IO,(aq) + 12 H"(aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. O-0 Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E° = Ov Round your answer to 2 decimal places.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
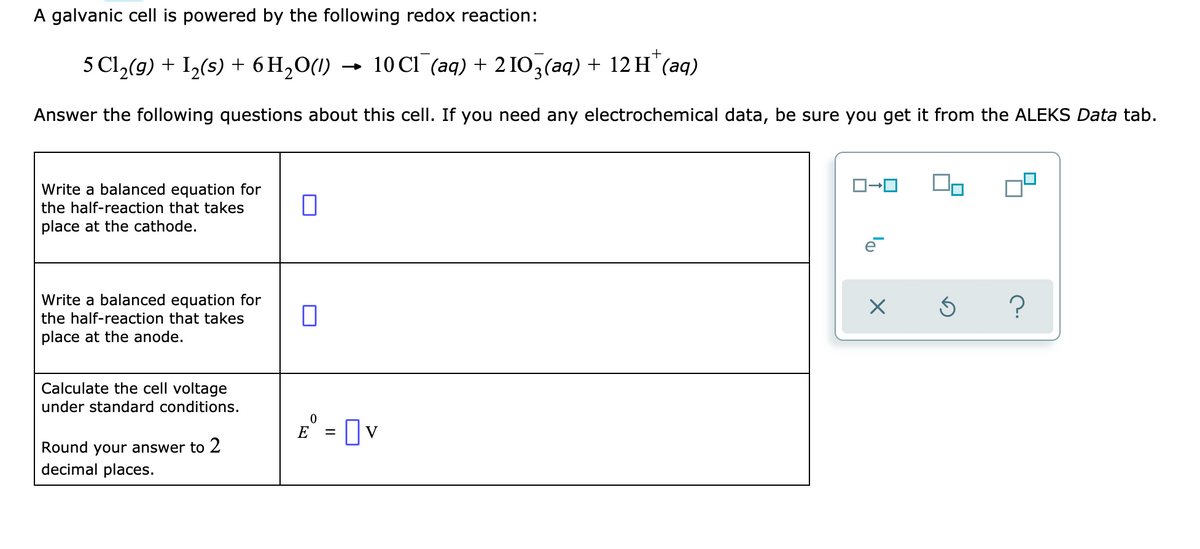
Transcribed Image Text:A galvanic cell is powered by the following redox reaction:
5 Cl,(9) + I2(s) + 6 H2O(1)
- 10 CI (aq) + 2 IO,(aq) + 12 H"(aq)
Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab.
Write a balanced equation for
the half-reaction that takes
place at the cathode.
Write a balanced equation for
the half-reaction that takes
place at the anode.
Calculate the cell voltage
under standard conditions.
E° = [ v
Round your answer to 2
decimal places.
lo
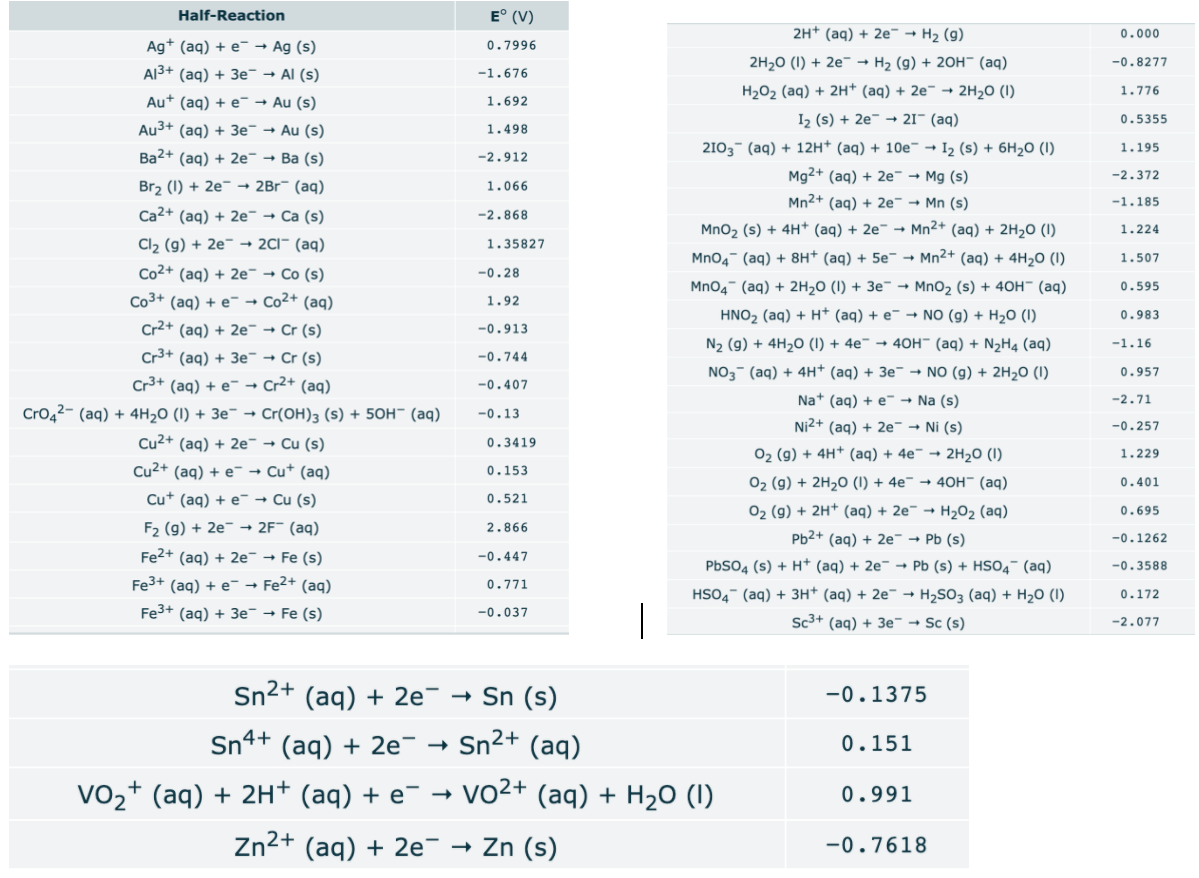
Transcribed Image Text:Half-Reaction
E° (V)
2H+ (aq) + 2e¯ → H2 (g)
0.000
Ag* (aq) + e¯ → Ag (s)
0.7996
2H20 (1) + 2e¯ - H2 (g) + 20H¯ (aq)
-0.8277
AI3+ (aq) + 3e¯ → Al (s)
-1.676
H202 (aq) + 2H* (aq) + 2e¯ → 2H20 (I)
1.776
Au+ (aq) + e¯→ Au (s)
1.692
I2 (s) + 2e- → 21¯ (aq)
0.5355
Au3+ (aq) + 3e¯ → Au (s)
1.498
Ba2+ (aq) + 2e¯ → Ba (s)
2103- (aq) + 12H* (aq) + 10e¯ → I2 (s) + 6H2O (1)
1.195
-2.912
Mg2+ (aq) + 2e¯ → Mg (s)
-2.372
Brz (1) + 2e¯ → 2Br¯ (aq)
1.066
Mn2+ (aq) + 2e¯ → Mn (s)
-1.185
Ca2+ (aq) + 2e¯ → Ca (s)
-2.868
MnO2 (s) + 4H+ (aq) + 2e¯ → Mn2+ (aq) + 2H20 (I)
1.224
Cl2 (g) + 2e¯ → 2C1¯ (aq)
1.35827
Mno4- (aq) + 8H+ (aq) + 5e¯ → Mn2+ (aq) + 4H20 (1)
1.507
Co2+ (aq) + 2e¯ → Co (s)
-0.28
Mn04 (aq) + 2H2O (I) + 3e¯ → MnO2 (s) + 40H¯ (aq)
0.595
Co3+ (aq) + e¯ → Co2+ (aq)
Cr2+ (aq) + 2e- → Cr (s)
1.92
HNO2 (aq) + H+ (aq) + e¯ → NO (g) + H2O (I)
0.983
-0.913
N2 (g) + 4H20 (1) + 4e¯ → 40H" (aq) + N½H4 (aq)
-1.16
Cr3+ (aq) + 3e¯ → Cr (s)
Cr3+ (aq) + e- → Cr2+ (aq)
-0.744
NO3- (aq) + 4H* (aq) + 3e¯ → NO (g) + 2H2O (1)
0.957
-0.407
Na+ (aq) + e - Na (s)
-2.71
Cro42- (aq) + 4H20 (I) + 3e¯ → Cr(OH)3 (s) + 50H" (aq)
-0.13
Ni2+ (aq) + 2e¯ → Ni (s)
-0.257
Cu2+ (aq) + 2e¯ → Cu (s)
0.3419
02 (g) + 4H* (aq) + 4e¯ → 2H20 (1)
1.229
Cu2+ (aq) + e- → Cu* (aq)
0.153
02 (g) + 2H20 (1) + 4e¯ → 40OH" (aq)
0.401
Cu* (aq) + e¯ → Cu (s)
0.521
02 (g) + 2H+ (aq) + 2e¯ → H2O2 (aq)
0.695
F2 (g) + 2e¯ → 2F¯ (aq)
2.866
Pb2+ (aq) + 2e - Pb (s)
-0.1262
Fe2+ (aq) + 2e¯ → Fe (s)
-0.447
PBSO4 (s) + H+ (aq) + 2e¯ → Pb (s) + HSO4 (aq)
-0.3588
Fe3+ (aq) + e¯ → Fe2+ (aq)
0.771
HSO4- (aq) + 3H+ (aq) + 2e¯ → H2SO3 (aq) + H20 (1)
0.172
Fe3+ (aq) + 3e¯ → Fe (s)
-0.037
Sc3+ (aq) + 3e - Sc (s)
-2.077
Sn2+ (aq) + 2e¯
Sn (s)
-0.1375
Sn4+ (aq) + 2e¯ →
Sn²+ (aq)
0.151
vo2+ (aq) + 2H+ (aq) + e¯ → vo2+ (aq) + H2O (1)
0.991
Zn2+ (aq) + 2e¯
- Zn (s)
-0.7618
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole