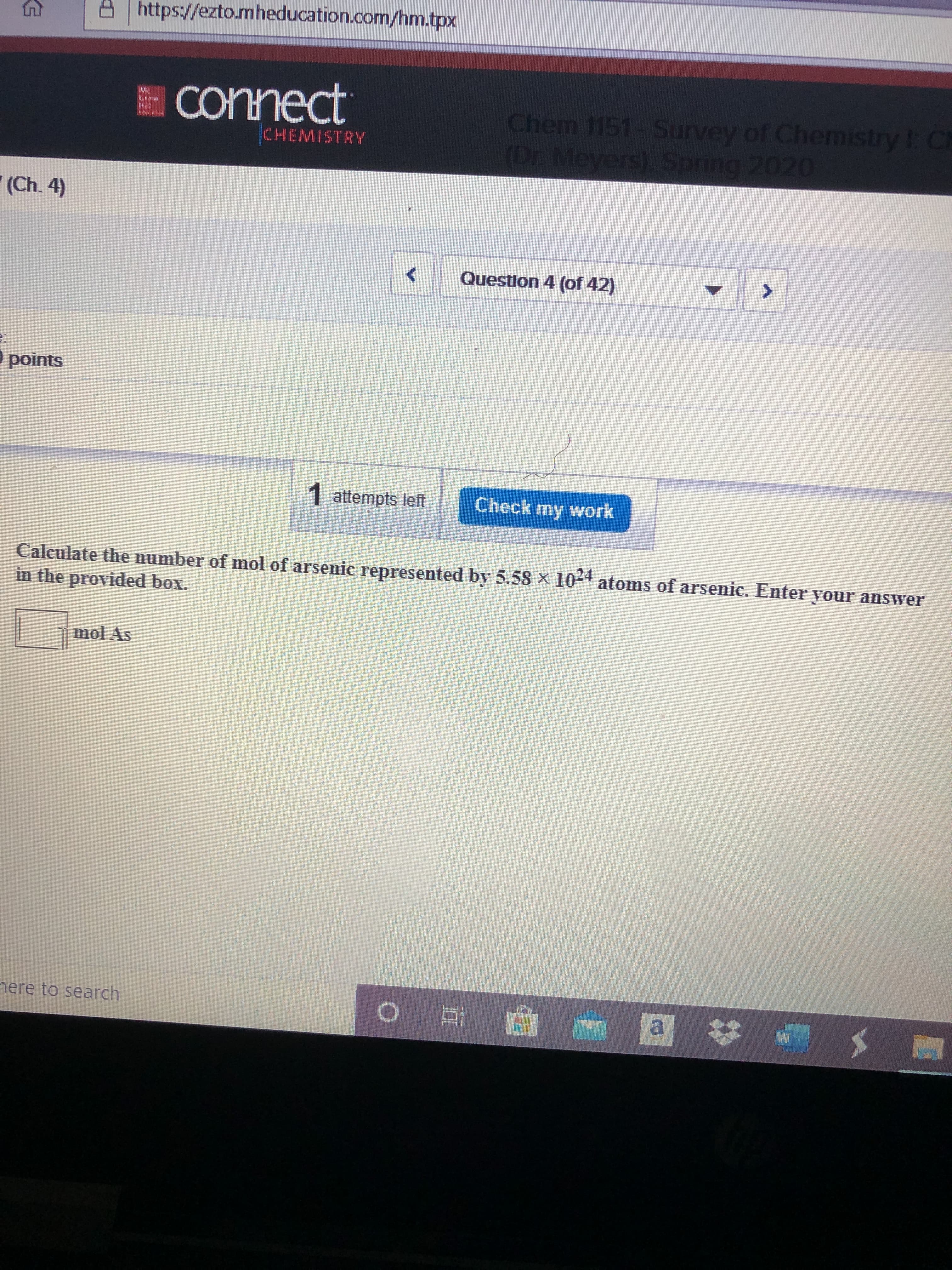
A https://ezto.mheducation.com/hm.tpx E Connect Chem 1151- Survey of Chemistry I: C (Dr. Meyers), Spring 2020 CHEMISTRY " (Ch. 4) Question 4 (of 42) points 1 attempts left Check my work Calculate the number of mol of arsenic represented by 5.58 x 104 atoms of arsenic. Enter your answer in the provided box. mol As nere to search a
A https://ezto.mheducation.com/hm.tpx E Connect Chem 1151- Survey of Chemistry I: C (Dr. Meyers), Spring 2020 CHEMISTRY " (Ch. 4) Question 4 (of 42) points 1 attempts left Check my work Calculate the number of mol of arsenic represented by 5.58 x 104 atoms of arsenic. Enter your answer in the provided box. mol As nere to search a
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 2CR: erhaps the most important concept in introductory chemistry concerns what a mole of a substance...
Related questions
Question
Transcribed Image Text:A https://ezto.mheducation.com/hm.tpx
E Connect
Chem 1151- Survey of Chemistry I: C
(Dr. Meyers), Spring 2020
CHEMISTRY
" (Ch. 4)
Question 4 (of 42)
points
1 attempts left
Check my work
Calculate the number of mol of arsenic represented by 5.58 x 104 atoms of arsenic. Enter your answer
in the provided box.
mol As
nere to search
a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning