(a) Illustrate a titration curve of the following structure. (Solid lines indicate covalent bonds) Indicate buffering regions (including size) and the isoelectric point, pl. Asn - Cys - Ala Lys-Cys - Phe (b) What is the predominant charge state at the following pH values? pH I, pH 4, pH 7, pH 11 (c) What would you expect to happen to your titration curve if you exposed this peptide to Beta- mercaptoethanol prior to titration? Describe the changes in the titration curve.
(a) Illustrate a titration curve of the following structure. (Solid lines indicate covalent bonds) Indicate buffering regions (including size) and the isoelectric point, pl. Asn - Cys - Ala Lys-Cys - Phe (b) What is the predominant charge state at the following pH values? pH I, pH 4, pH 7, pH 11 (c) What would you expect to happen to your titration curve if you exposed this peptide to Beta- mercaptoethanol prior to titration? Describe the changes in the titration curve.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 37QAP: Given three acid-base indicators—methyl orange (end point at pH 4), bromthymol blue (end point at...
Related questions
Question
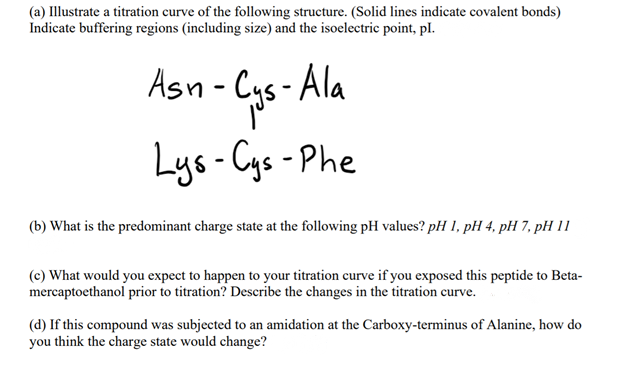
Transcribed Image Text:(a) Illustrate a titration curve of the following structure. (Solid lines indicate covalent bonds)
Indicate buffering regions (including size) and the isoelectric point, pl.
Asn - Cys - Ala
Lys-Cys -Phe
(b) What is the predominant charge state at the following pH values? pH 1, pH 4, pH 7, pH 11
(c) What would you expect to happen to your titration curve if you exposed this peptide to Beta-
mercaptoethanol prior to titration? Describe the changes in the titration curve.
(d) If this compound was subjected to an amidation at the Carboxy-terminus of Alanine, how do
you think the charge state would change?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning