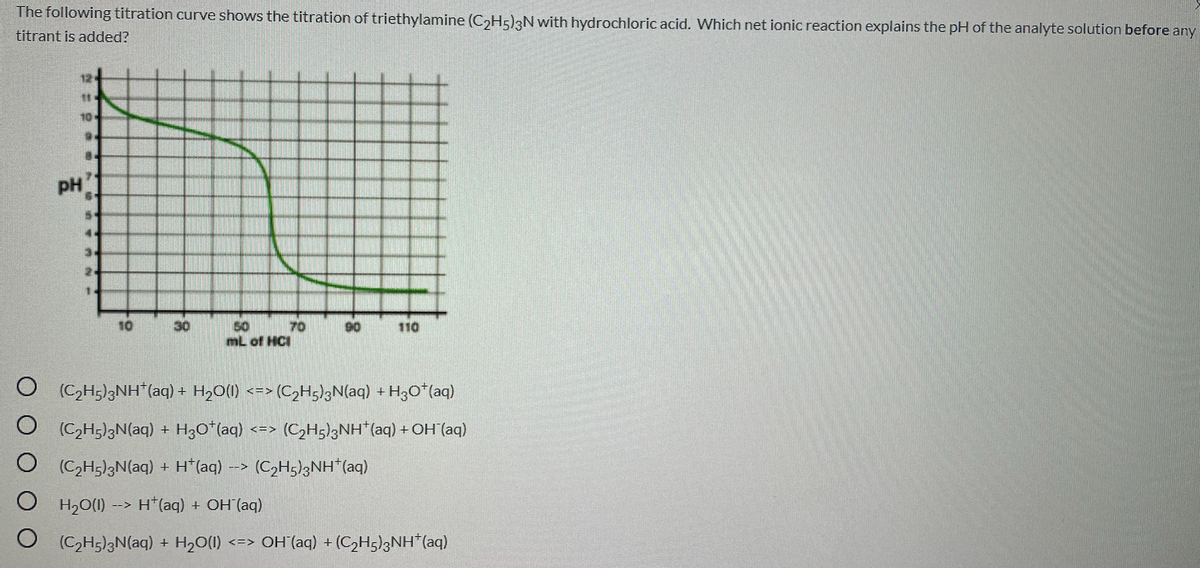
The following titration curve shows the titration of triethylamine (C,H5)3N with hydrochloric acid. Which net ionic reaction explains the pH of the analyte solution before any titrant is added? 12 10 6. PH 4. 1. 10 50 mL of HCI 30 70 90 110 (CH;)3NH"(aq) + H,0(1) <=> (C,Hg)gN(aq) + H3O*(aq) O C,H;)3N(aq) + H3O*(aq) (CH5)3NH*(aq) +OH (aq) <=> (C2H5)3N(aq) + H*(aq) -> (C,H5)3NH"(aq) O H2O(1) H*(aq) + OH (aq) --> (C2H;)3N(aq) + H,0(1) <=> OH (aq) + (CH5)3NH*(aq)
Q: A 0.3654 g portion of pure formic acid (CH2O2, FW=46.03 g/mol, Ka=1.77 x 10-4) is dissolved in 50.00…
A: Given that, Mass of formic acid = 0.3654 g Molecular mass of formic acid = 46.03 g/mol Volume of…
Q: Write the chemical reactions whose equilibrium constants are K, and K, for imidazole (C,H,N,) and…
A: Imidazole (C3H4N2) is a weak base whose conjugate acid is imidazole hydrochloride (C3H4N2H+Cl-).
Q: solution of 0.138 M aspartic acid, the charge neutral form of the amino acid, is titrated with…
A: In an acid - base titration, there is a 1:1 acid base stoichiometry, so the equivalence point where…
Q: Phenylacetic acid (C6H5CH2CO2H) is a weak monoprotic acid with Ka = 4.90 × 10−5. What is the pH that…
A: 2.56 g of phenylacetic acid is mixed with 1.59 g of potassium phenylacetate and the solution is…
Q: An analytical chemist is titrating 173.4 mL of a 0.6400 M solution of trimethylamine ((CH,) N with a…
A: When a strong acid is added to a weak base, then a buffer solution is formed. pOH of buffer solution…
Q: I need help understanding why the ratio of Acetate:Acetic acid = 3.5:1 and why the total amount…
A:
Q: Many chemical reactions are carried out in the presence of buffers. Use the Henderson-Hasselbalch…
A: Buffer solution : it is a solution which resists in change in pH of solution when a small amount of…
Q: 4. Tris(hydroxymethyl)aminomethane [(HOCH2)3CNH2–Tris, or THAM] is a weak base frequently used to…
A: Buffers are the mixtures that resists change in pH when small amount of acid or base is added to it.…
Q: The base ionization constant of ethylamine (C2H5NH2) in aqueous solution is Kb= 6.41 × 10-4 at 25°C.…
A: The reaction of Ethylamine when titrated with HCl is as follows:
Q: Given the following experimental data, calculate the molarity and the pKa of the acid analyte (HA)…
A:
Q: Given the following experimental data, calculate the molarity and the pKa of the acid analyte (HA)…
A: Solution is given below in next step in jpeg format
Q: The normal pH of blood is 7.40 6 0.05 and is controlled in part by the H2CO3/HCO3- buffer system.(a)…
A: Given: The pH of the blood= 7.20 The Ka for the carbonic acid=4.5 * 10-7.
Q: 4. A 0.1036-g sample containing only BaC2 and NaCl is dissolved in 50 mL of distilled water.…
A: Given data Mass of sample mixture [BaCl2 + NaCl] = 0.1036 g Volume of distilled water= 50 mL…
Q: 40.00mL of a 0.100M benzoic acid (Bz) solution are mixed with 20.00mL of 0.100M sodium hydroxide…
A: The Henderson Hasselbalch equation for acidic buffer is given as :
Q: 4. Tris(hydroxymethyl)aminomethane [(HOCH2);CNH2–Tris, or THAM] is a weak base frequently used to…
A: 4. Solution - pH = pH is a scale used to specify the acidity or basicity of an aqueous solution.…
Q: . 25.0 ml of a 0.100 M trimethylamine (CH3);N (a base like ammonia) Kb = 6.3×10-5, are transferred…
A: Given that 25.0 ml of a 0.100 M trimethylamine Kb = 6.3x105 0.100 M of HCl
Q: Write the chemical reactions whose equilibrium constants are K, and K, for imidazole (C, H, N,) and…
A:
Q: What are the molar concentrations of acetic acid (CH,COOH) and sodium acetate (CH, COONA) in an…
A: Buffer solution: buffer solution is an aqueous solution consisting of a mixture of a weak acid(HA)…
Q: An analytical chemist is titrating 187.6 mL of a 0.4000M solution of trimethylamine ((CH3),N) with a…
A: Given volume of (CH3)3N = 187.6 mL Molarity of (CH3)3N = 0.4000 M Molarity of HNO3 = 0.7000 M…
Q: Calculate the volume, in milliliters, of 0.170 M NaOH that must be added to 305 mL 0.0409 M…
A:
Q: Benzoic acid (C₂H5CO₂H), a weak acid, has a dissociation constant of K=6.3x10^-5. 1. Write the…
A:
Q: What molar ratio of salt to acid would be required to prepare a buffer solution with a pH of 5.9?…
A:
Q: 4. A 0.1036-g sample containing only BaCl2 and NaCl is dissolved in 50 mL of distilled water.…
A: Given, Mass of sample = 0.1036 g Volume of water = 50 mL Concentration of AgNO3 solution = 0.07916 M…
Q: 3. Uh oh, the labels fell off the bottles of chloroaniline (FW 127.57). Again. There are three of…
A: (a) The reaction that occurs during the titration is:…
Q: 3. (a) How many grams of sodium succinate (140 g/mol) and disodium succinate (162 g/mol) must be…
A: A buffer solution of 50Mm with a pH 6, has to be prepared using sodium succinate and disodium…
Q: ou have been asked to prepare 250.0 mL of a buffer solution with a pH of 8.88 at room temperature.…
A: Given: Volume of buffer = 250.0 mL = 0.250 L (Since 1 L = 1000 mL) pH…
Q: Write the values of Y and X. H2SO4 CH;CH=CH2 + H2O CH3CH-CH2 H HỌ 2-propanol an alcohol The pKa of a…
A: The acid dissociation constant Ka is the representation of acidic strength of a solution and pKa is…
Q: It has been found the azide (N3-) forms an "insoluble" compound when in the presence of lead, which…
A: Pb(N3)2 = Pb2+ + 2N3- if solubility is s then: Pb(N3)2 = Pb2+ + 2N3-…
Q: Calculate the pH at the following points in a titration of 40. mL (0.040 L) of 0.145 M…
A: 4-chlorobenzoic acid is a weak acid. The chemical equation for the given question may be written as:…
Q: What is the molar solubility of Ag2CO3 in an aqueous solution that is buffered to a pH of 2.37?…
A: Given: pH = 2.37 Ksp for Ag2CO3 = 8.1 x 10-12 K1 H2CO3 = 4.45x10-7 K2 for HCO3- = 4.69x10-11 Kw =…
Q: An analytical chemist Is titrating 108.4 mL of a 0.2200M solution of plperidine (CH10 NH) with a…
A:
Q: .00 mL of a nitric acid solution of unknown concentration is pipetted into a 125-mL Erlenmeyer ask…
A: Molarity is defined as “the mole of the solute per unit volume of the solution”. It is represented…
Q: Tris(hydroxymethyl)aminomethane [(HOCH2)3CNH2, or "Tris" for short] acts as a Brønsted- Lowry base…
A:
Q: A chemist generates the titration curve shown by slowly 12 adding 1.0 M sodium hydroxide (NaOH) to…
A: Titration curve is the graph in which volume of titrant plotted against pH.
Q: Tris or tris(hydroxymethyl)aminomethane is an organic buffering reagent most often used in nucleic…
A: Solution is given below in next step
Q: 6. If 25.1 mL of 0.109M acid with a pKa of 5.27 is titrated with 0.108 M NaOH solution, what is the…
A: The volume of acid V1=25.1 mL The concentration of acid C1=0.109 M The strength of an acid is pKa =…
Q: Generate a titration curve for 25.0 mL of 0.7233 M H3PO4, with 1.256 M NaOH as the titrant, given…
A: The titration curve for the titration of 25.0 mL of 0.7233 M H3PO4 versus 1.256 M NaOH solution is…
Q: How many grams of dipotassium succinate trihydrate (K2C4H4O4-3H2O, MW = 248.32 g/mol) must be added…
A: Given, The pH of the solution is 5.813. The molarity of succinic acid is 0.0338 M. pKa2 is 5.636.…
Q: Propanoic acid (CH3CH2COOH) has a Ka of 1.3×10–5. FW(propanoic acid) = 74.08 g/mol; FW(calcium…
A: Buffers are generally good over the range pH = pKa ± 1. For example, the acetate buffer would be…
Q: A 100 ml of 0.100 M phosphate buffer solution with a pH of 8.10 is to be prepared by adding 1.00 M…
A: The required pH of the buffer is 8.10, which is closest to the pKa2 value. H2PO4(aq) ⇌ HPO4-(aq) +…
Q: The concentration of Cl– in a 100.0-mL sample of water drawn from a fresh water acquifer suffering…
A: Given data, Volume of sample = 100mL = 0.1L Molarity of Hg(NO3)2 = 0.0516 M Volume of Hg(NO3)2 =…
Q: When a drop (taken to be 0.20 cm3) of 1.0 M HCI(aq) is added to 25 cm3 of pure water, the resulting…
A: Calculate the number of moles of HCl, ethanoic acid and sodium ethanoate. Number of moles =…
Q: Tris(hydroxymethyl)aminomethane [(HOCH2)3CNH2—Tris, or THAM] is a weak base frequently used to…
A: Given, pH of buffer =7.4 pKa =8.08 pH of any buffer solution can be calculated by…
Q: To rule out metabolic acidosis in a patient's blood sample, the total carbon dioxide content should…
A: Henderson-Hasselbach equation. this equation shows the relationship between pH of a solution, the…
Q: An analytical chemest is titrating 150.6 ml. of a 0.6600 M solution of dhethylamine (C,H,), NH) with…
A:
Q: To purify a certain protein, you were required to prepare a 0.10 M glycine buffer at pH 9.4.…
A: The one at pH 9.0 has a concentration of 10^-9 of H+, and the one at pH 10.0 has a concentration of…
Q: A primary amine R-NH2 has pKb = 5.65 at 25°C. A buffer solution is made by adding 66.0 mL of 1.55…
A:
Q: To create a buffer solution, 40.00mL of a 0.100M benzoic acid (Bz) solution are mixed with 20.00mL…
A: This means potential of hydrogen and to calculate this we have to know the negative logarithmic…
Q: Please answer 13.3 and 13.4
A:
Step by step
Solved in 2 steps with 1 images

- When a drop (taken to be 0.20 cm3) of 1.0 M HCI(aq) is added to 25 cm3 of pure water, the resulting hydroniumion concentration rises to 0.0080 mol dm- 3 and so the pH changes from 7.0 t o 2.1, a big change. Now suppose the drop is added to 25 cm3 of an ethanoate buffer solution that is 0.040 MNaCH3CO2(aq) and 0.080 M CH3COOH(aq). What will be the change in pH?The concentration of Cl– in a 300.0-mL sample of water drawn from a fresh water acquifer suffering from encroachment of sea water, was determined by titrating with 0.0516 M Hg(NO3)2. The sample was acidified and titrated to the diphenylcarbazone end point, requiring 6.18 mL of the titrant. Report the concentration of Cl–in parts per millionYou have performed an iodimetric titration using a commercial vitamin C tablet. Based on the following information below, calculate the %(w/w) of vitamin C(MM=176.16 g/mol) in the tablet: Mass of tablet dissolved in 250.0 mL: 5.422 g Aliquot volume of sample titrated: 25.00 mL Concentration of KIO3: 0.023 M Final burrette volume: 41.31 mL Initial burrette volume: 8.89 mL Blank volume: 0.14 mL
- ) A truck driver carrying a load of lead nitrate (Pb(NO3)2) lost control of his semi- truck after hitting a patch of ice and crashed the truck into Blue Lake, which was right next to the highway. Despite the best efforts of the emergency workers, several of the crates containing lead nitrate were damaged, and the highly soluble compound dissolved immediately. Assuming the lake is initially at circumneutral pH (7), and that the spill resulted the in a total lead concentration of 10-3 M within the lake. Based on the following information, will PbO(s) precipitate out of Blue Lake. Assume all lead nitrate dissociates into Pb+2 and NO3-, no other sources of lead exist in the lake and that no other reactions besides the equations shown below occur. PbO(s) + 2H+ ⇌ PbO(s) + H+ ⇌ PbO(s) + H2O ⇌ PbO(s) + 2H2O ⇌ Pb2+ + H2O PbOH+ + H2O Pb(OH)2o Pb(OH)3- + H+ logKs0/ksp =14 logK1=3.4 logK2=-0.5 logK3=-12The protein content of wheat flour can be determined reasonably accurately by multiplying the percentage of nitrogen present by 5.7. A 2.06-g sample of flour was taken through a Kjeldahl procedure and the ammonia produced was distilled into a boric acid solution. If this solution required 34.70 mL of 0.174 N HCl for titration to the methyl red end point, what is the a) % Nitrogen and b) % protein in the flour? (Use 1:1 stoich ratio between N and HCl)The Tris buffer system is commonly used in biochemistry as its pKa of 8.1 allows it to buffer close to physiological pH. (CH2OH)3CNH2 + H+ Û (CH2OH)3CNH3+ What concentrations of Tris and Tris H+ are obtained in a 200 mL solution at pH 8.20 containing 6.1g of Tris? The molecular mass of Tris is 121.1 gmol-1 Please do this step by step thank you!
- The liquid part of the Moderna vaccine contains a Tris (tromethamine) buffer. The purpose of the buffer is to make the pH level of the vaccine near to that of the human body and also to maintain the integrity of the mRNA component.In a laboratory scale, a biochemist was asked to study the structure of the mRNA component of the Moderna vaccine. To do so, they need to first prepare 500.0 mL of 1.000 M of the Tris buffer solution of pH 7.5000. The available reagents in the lab are the following:Tris base MW = 121.14 g/mol pKa = 8.07Tris-HCl MW = 157.56 g/mol Calculate the amounts of tris base and tris-HCl needed to prepare the buffer solution.A 10.00cm3 portion of the 100.00cm3 HCl solut was taken from the volumetric flask and was titrated with KOH (aq). It was neeutralized by 24.35cm3 of potassium hydroxide od concentration 0.0500moldm-3. Calculate the concentrat of the original concentrated hydrochloric acid in moldm-3.For the synthesis of maroon dye, Taylor needs to use a primary amine with a pH between 3.00 and 4.00 for optimum yield. In one of her trials, she dissolved 8.22 g of anilinium chloride, C6H5NH3Cl (MM = 129.58 g/mol) in distilled water to make 250.0 mL solution. Given that Kb of aniline (C6H5NH2) is 4.29 x 10-10, calculate the pH of the salt solution. Yes or No. Will Taylor obtain high yield of maroon using C6H5NH3Cl?'
- Ammonium acetate buffer is used as an important reagent for studying molecular biology, biological buffers, reagents and DNA and RNA purification. Ammonium acetate (0.24M) in combination with cold 66% (v/v) ethanol quantitatively precipitated RNA from very dilute solutions (greater than or equal to μg/ml) after centrifugation. Ammonium acetate (CH3COONH4) is also a salt of weak acid (CH3COOH) and weak base (NH4OH). The Ka and Kb are equal to 1.8 x10-5. The pH of this salt solution will be, a. pH > 7 b. pH ≈ 7 c. pH < 7 d. both cation and anion don’t hydrolyze. e. none of the above.Following titration analysis, a sample of a fruit juice was found to contain 8.9 mg cm-3 ascorbic acid. Given that the Mr value for ascorbic acid is 176.1 Da, calculate the value of the ascorbic acid concentration expressed in units of mmols dm-3 then enter itProperties of a Buffer The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. The amino group of glycine, which has a of 9.6, can exist either in the protonated form or as the free base , because of the reversible equilibrium In what pH range can glycine be used as an effective buffer due to its amino group? In a 0.1 m solution of glycine at pH 9.0, what fraction of glycine has its amino group in the form? How much 5 m KOH must be added to 1.0 L of 0.1 m glycine at pH 9.0 to bring its pH to exactly 10.0? When 99% of the glycine is in its form, what is the numerical relation between the pH of the solution and the of the amino group? Please answer all of these questions, especially C please write in detail.

