(a) Imagine that a Born-Haber cycle is being constructed by a colleague, to determine the lattice energy of a hypothetical magnesium halide salt, where X is the halide ion. The colleague makes the following list: First and second ionisations of Mg; 737 and 1451 Sublimation energy of Mg; 147 Electron affinity of X; 200 Atomisation enthalpy of X₂; 400 Enthalpy of formation of MgX₂; -450 2+ 2e + Mg(+2X(g) 2+ 2e + Mg() + X₂ (8) + Mg(g) + X₂ (8) a Mg(g) + X₂ (8) 1451 Mg(s) + X₂ (8) 147 MgX2 (s) с 2+ Mg(g) +2X(g) d Sadly the colleague becomes discouraged and leaves the document incomplete, simply drawing boxes around unknown quantities. Complete the calculation by writing down the boxed quantities a-f on your answer paper. You may assume that the units are kJ
(a) Imagine that a Born-Haber cycle is being constructed by a colleague, to determine the lattice energy of a hypothetical magnesium halide salt, where X is the halide ion. The colleague makes the following list: First and second ionisations of Mg; 737 and 1451 Sublimation energy of Mg; 147 Electron affinity of X; 200 Atomisation enthalpy of X₂; 400 Enthalpy of formation of MgX₂; -450 2+ 2e + Mg(+2X(g) 2+ 2e + Mg() + X₂ (8) + Mg(g) + X₂ (8) a Mg(g) + X₂ (8) 1451 Mg(s) + X₂ (8) 147 MgX2 (s) с 2+ Mg(g) +2X(g) d Sadly the colleague becomes discouraged and leaves the document incomplete, simply drawing boxes around unknown quantities. Complete the calculation by writing down the boxed quantities a-f on your answer paper. You may assume that the units are kJ
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 38P: The percent ionic character of the bonds in several interhalogen Molecules (as estimated from their...
Related questions
Question
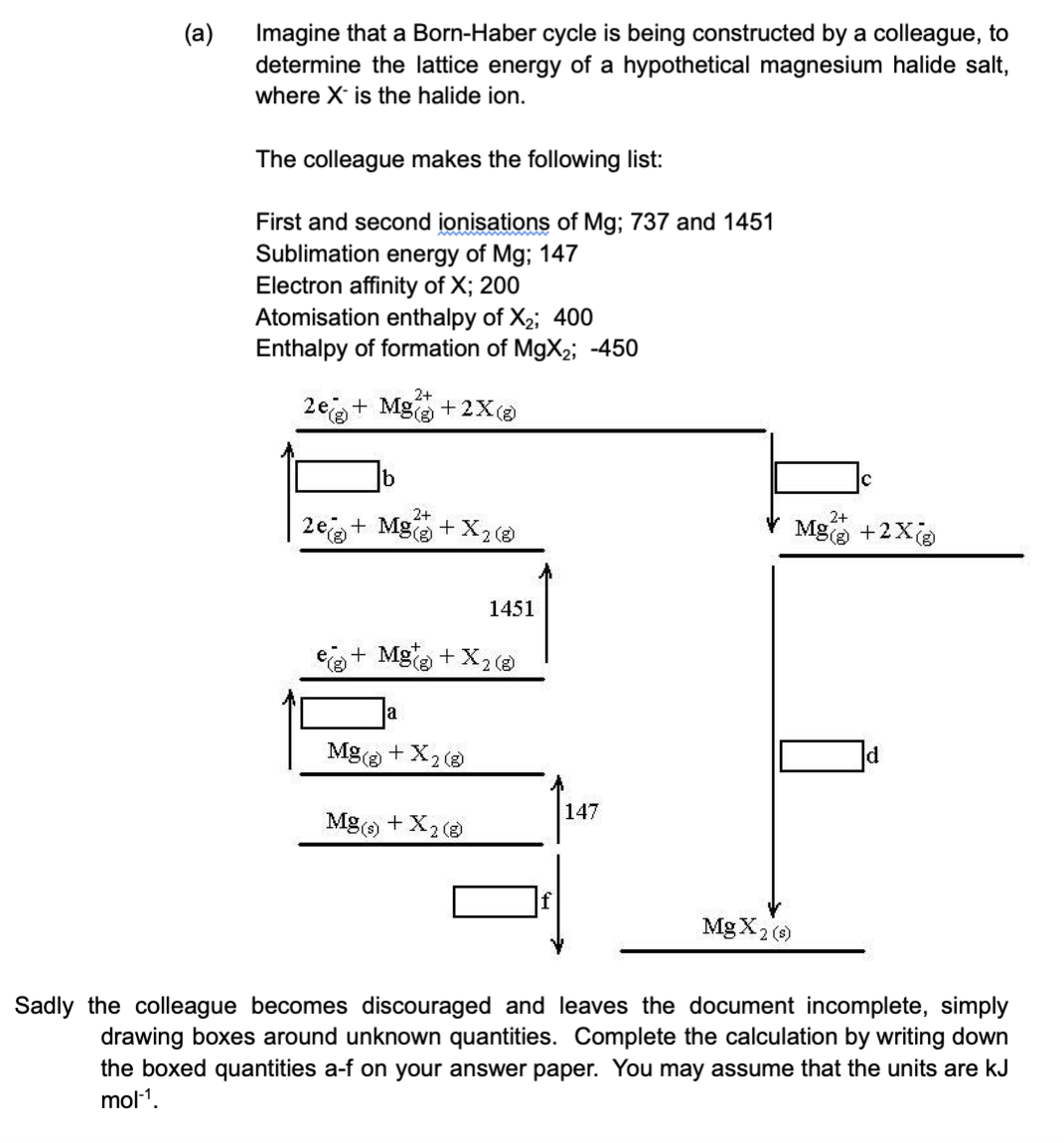
Transcribed Image Text:(a)
Imagine that a Born-Haber cycle is being constructed by a colleague, to
determine the lattice energy of a hypothetical magnesium halide salt,
where X is the halide ion.
The colleague makes the following list:
First and second ionisations of Mg; 737 and 1451
Sublimation energy of Mg; 147
Electron affinity of X; 200
Atomisation enthalpy of X₂; 400
Enthalpy of formation of MgX₂; -450
2+
2e+ Mg(+2X (8)
b
2+
2e + Mg(
+ X2 (8)
a
+ Mg(g) + X₂ (8)
1451
Mg(g) + X₂ (8)
Mg(s) + X2 (8)
f
147
2+
Mg(g) +2X(g)
MgX2 (s)
d
Sadly the colleague becomes discouraged and leaves the document incomplete, simply
drawing boxes around unknown quantities. Complete the calculation by writing down
the boxed quantities a-f on your answer paper. You may assume that the units are kJ
mol-¹.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,