a) In each of the following, predict the directing effects. Indicate position(s) on the ring with (an) arrow(s) where an electrophile might be directed. Strong Activator Weak Activator Strong Activator b) Strong Deactivatod Strong Deactivalor Weak Activator Weak Activator Strong Activator Strong Deactivator Weak Astivator
a) In each of the following, predict the directing effects. Indicate position(s) on the ring with (an) arrow(s) where an electrophile might be directed. Strong Activator Weak Activator Strong Activator b) Strong Deactivatod Strong Deactivalor Weak Activator Weak Activator Strong Activator Strong Deactivator Weak Astivator
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter19: Eas: Electrophilic Aromatic Substitution
Section: Chapter Questions
Problem 21E
Related questions
Question
Transcribed Image Text:9:11 1
CH237 Pre-recita...
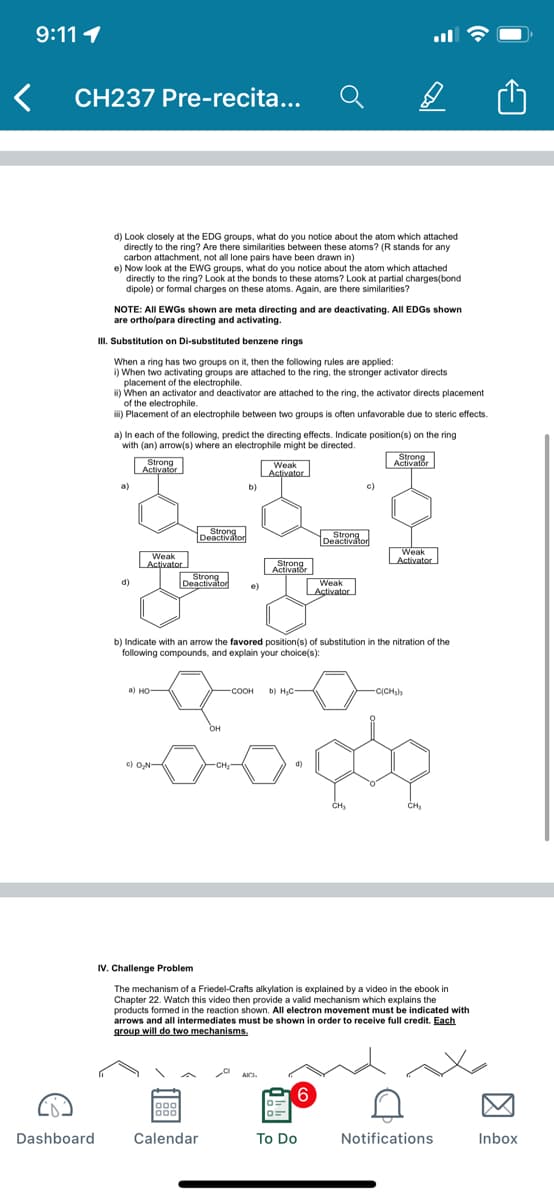
d) Look closely at the EDG groups, what do you notice about the atom which attached
directly to the ring? Are there similarities between these atoms? (R stands for any
carbon attachment, not all lone pairs have been drawn in)
e) Now look at the EWG groups, what do you notice about the atom which attached
directly to the ring? Look at the bonds to these atoms? Look at partial charges(bond
dipole) or formal charges on these atoms. Again, are there similarities?
NOTE: All EWGS shown are meta directing and are deactivating. All EDGS shown
are ortho/para directing and activating.
II. Substitution on Di-substituted benzene rings
When a ring has two groups on it, then the following rules are applied:
i) When two activating groups are attached to the ring, the stronger activator directs
placement of the electrophile.
i) When an activator and deactivator are attached to the ring, the activator directs placement
of the electrophile.
i) Placement of an electrophile between two groups is often unfavorable due to steric effects.
a) In each of the following, predict the directing effects. Indicate position(s) on the ring
with (an) arrow(s) where an electrophile might be directed.
Strong
Actiyator
Weak
Activator
Strong
Activator
a)
b)
c)
Strong
Deactivator
Strong
d.
Deactivalor
Weak
Weak
Activator
Activator
weak
Strong
Activator
Strong
Deactivator
Weak
Activator
d)
e)
b) Indicate with an arrow the favored position(s) of substitution in the nitration of the
following compounds, and explain your choice(s):
а) но—
COOH
соон
b) H,C-
-C(CH,h
OH
c) O,N
-CH
CH
CH
IV. Challenge Problem
The mechanism of a Friedel-Crafts alkylation is explained by a video in the ebook in
Chapter 22. Watch this video then provide a valid mechanism which explains the
products formed in the reaction shown. All electron movement must be indicated with
arrows and all intermediates must be shown in order to receive full credit. Each
group will do two mechanisms.
ACI.
6
Dashboard
Calendar
To Do
Notifications
Inbox
因
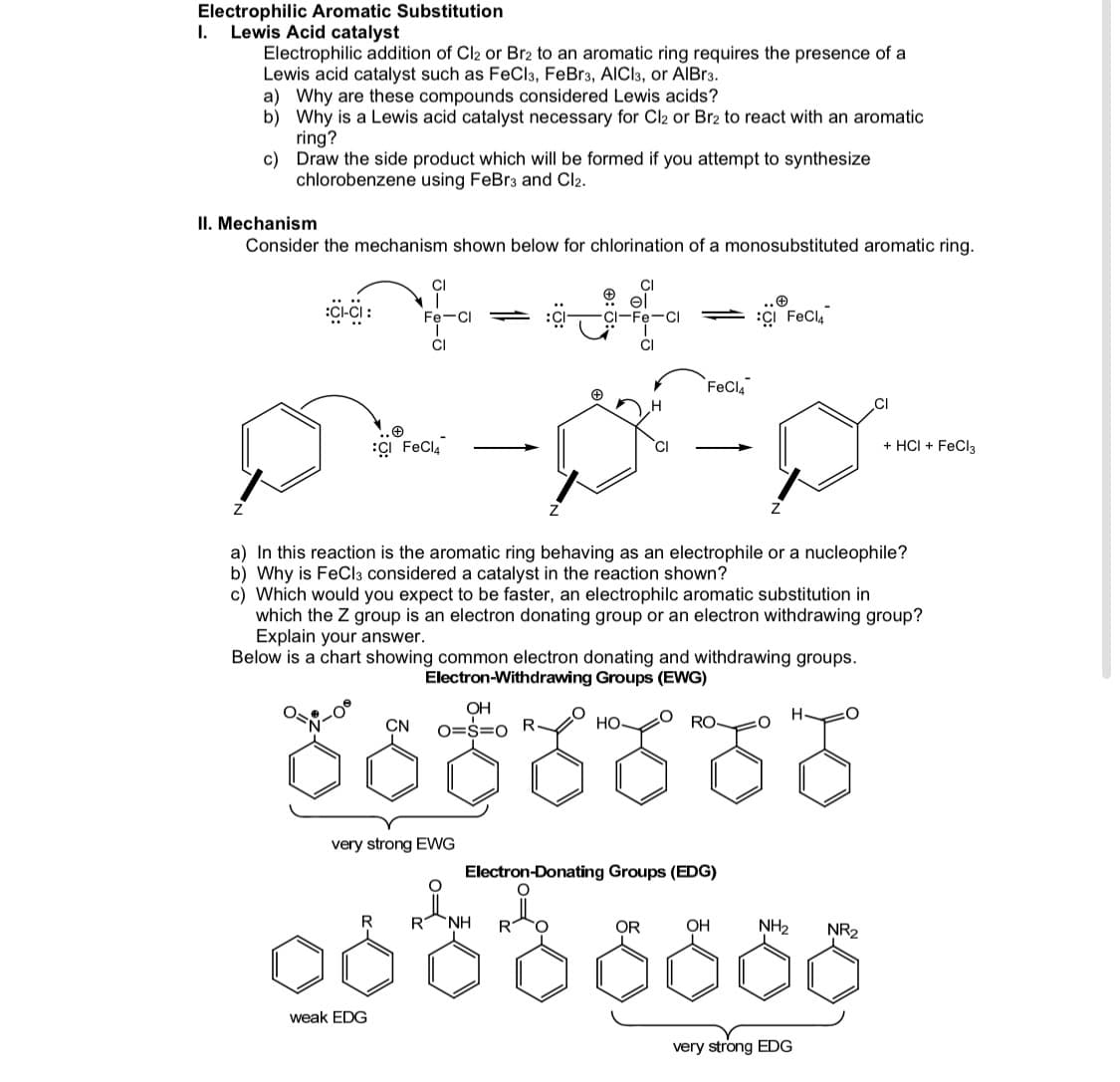
Transcribed Image Text:Electrophilic Aromatic Substitution
I.
Lewis Acid catalyst
Electrophilic addition of Cl2 or Br2 to an aromatic ring requires the presence of a
Lewis acid catalyst such as FeCla, FeBr3, AICI3, or AIBR3.
a) Why are these compounds considered Lewis acids?
b) Why is a Lewis acid catalyst necessary for Cl2 or Br2 to react with an aromatic
ring?
c) Draw the side product which will be formed if you attempt to synthesize
chlorobenzene using FeBr3 and Cl2.
II. Mechanism
Consider the mechanism shown below for chlorination of a monosubstituted aromatic ring.
CI
CI-Fe-C
ÇI FeCl,
Fe-CI
CI
FeCla
CI
ÇI FeCla
+ HCI + FeCl3
a) In this reaction is the aromatic ring behaving as an electrophile or a nucleophile?
b) Why is FeCla considered a catalyst in the reaction shown?
c) Which would you expect to be faster, an electrophilc aromatic substitution in
which the Z group is an electron donating group or an electron withdrawing group?
Explain your answer.
Below is a chart showing common electron donating and withdrawing groups.
Electron-Withdrawing Groups (EWG)
రంరంరం
OH
CN
O=S=0
НО
RO
very strong EWG
Electron-Donating Groups (EDG)
R
R NH
R
OR
OH
NH2
NR2
weak EDG
very strong EDG
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning