A laboratory technician dissolved 6.797 g of compound W (a non-electrolyte) in 15.00 mL of ethylene glycol in a boiling tube and heated the sample. If the boiling point of the mixture was found to be 201.4 °C, what is the molar mass of W? A buffer is prepared by adding together 600 mL of 1.64 M hydrocyanic acid and 850 mL of 0.98 M sodium cyanide. Given that the new pH of the buffer is 9.01 after 25 mL of sulfuric acid is added, calculate the concentration of the sulfuric acid.
A laboratory technician dissolved 6.797 g of compound W (a non-electrolyte) in 15.00 mL of ethylene glycol in a boiling tube and heated the sample. If the boiling point of the mixture was found to be 201.4 °C, what is the molar mass of W? A buffer is prepared by adding together 600 mL of 1.64 M hydrocyanic acid and 850 mL of 0.98 M sodium cyanide. Given that the new pH of the buffer is 9.01 after 25 mL of sulfuric acid is added, calculate the concentration of the sulfuric acid.
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
H8.
Transcribed Image Text:+
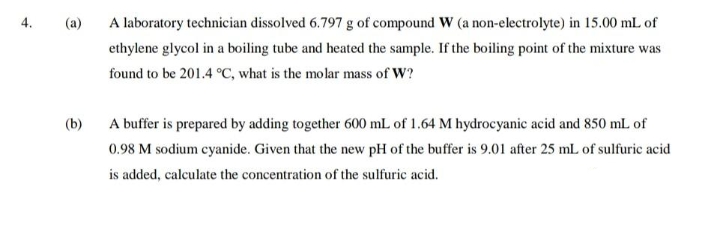
(a)
A laboratory technician dissolved 6.797 g of compound W (a non-electrolyte) in 15.00 mL of
ethylene glycol in a boiling tube and heated the sample. If the boiling point of the mixture was
found to be 201.4 °C, what is the molar mass of W?
(b)
A buffer is prepared by adding together 600 mL of 1.64 M hydrocyanic acid and 850 mL of
0.98 M sodium cyanide. Given that the new pH of the buffer is 9.01 after 25 mL of sulfuric acid
is added, calculate the concentration of the sulfuric acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning