A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an Part A anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: If the following elements were to form ions, they would attain the same number of electrons as which noble gas? Drag the appropriate elements to their respective bins. Group Tendency Charge • View Available Hint(s) 1 (1A) Lose one electron +1 2 (2A) Lose two electrons +2 Reset Help 13 (3A) Lose three electrons +3 15 (5A) Gain three electrons -3 16 (6A) Gain two electrons -2 17 (7A) Gain one electron -1 Li o|C K Mg Sr As 18 (8A) Rarely gain or lose electrons Не Ne Ar Kr
A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an Part A anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: If the following elements were to form ions, they would attain the same number of electrons as which noble gas? Drag the appropriate elements to their respective bins. Group Tendency Charge • View Available Hint(s) 1 (1A) Lose one electron +1 2 (2A) Lose two electrons +2 Reset Help 13 (3A) Lose three electrons +3 15 (5A) Gain three electrons -3 16 (6A) Gain two electrons -2 17 (7A) Gain one electron -1 Li o|C K Mg Sr As 18 (8A) Rarely gain or lose electrons Не Ne Ar Kr
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section: Chapter Questions
Problem 98CP
Related questions
Question
Please answer question 9 part A and C
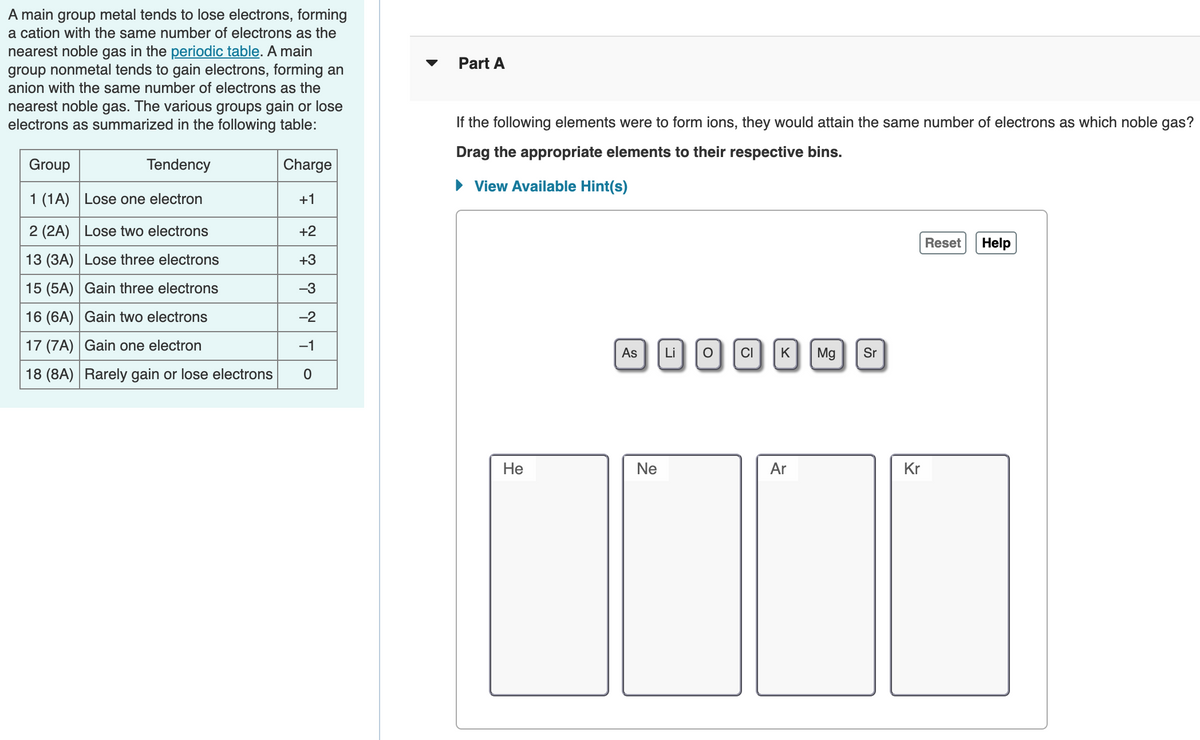
Transcribed Image Text:A main group metal tends to lose electrons, forming
a cation with the same number of electrons as the
nearest noble gas in the periodic table. A main
group nonmetal tends to gain electrons, forming an
anion with the same number of electrons as the
Part A
nearest noble gas. The various groups gain or lose
electrons as summarized in the following table:
If the following elements were to form ions, they would attain the same number of electrons as which noble gas?
Drag the appropriate elements to their respective bins.
Group
Tendency
Charge
• View Available Hint(s)
1 (1A) Lose one electron
+1
2 (2A) |Lose two electrons
+2
Reset
Help
13 (3A) Lose three electrons
+3
15 (5A) Gain three electrons
-3
16 (6A) Gain two electrons
-2
17 (7A) Gain one electron
-1
Mg
Sr
As
Li
CI
K
18 (8A) Rarely gain or lose electrons
Не
Ne
Ar
Kr
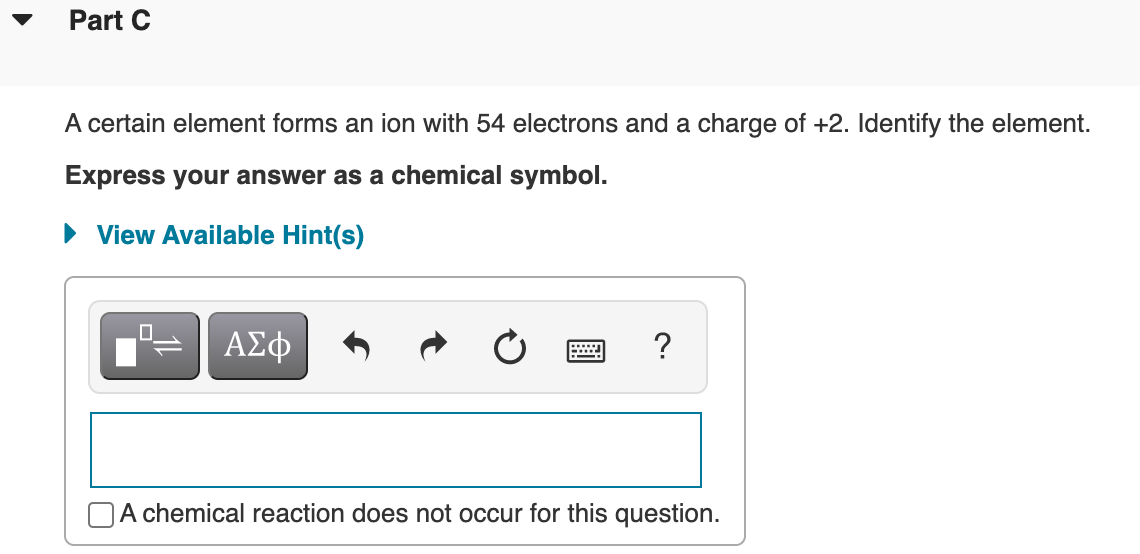
Transcribed Image Text:Part C
A certain element forms an ion with 54 electrons and a charge of +2. Identify the element.
Express your answer as a chemical symbol.
• View Available Hint(s)
?
A chemical reaction does not occur for this question.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning