A membrane that is permeable to Na+ but not Cl or water separates two compartments (see figure). Side A is filled with 1000 mOsM NaCl and side B is fillec with 10 mOsM NaCl. Electrodes from a voltmeter are placed in each compartment and side A is set as ground (0 mV).
A membrane that is permeable to Na+ but not Cl or water separates two compartments (see figure). Side A is filled with 1000 mOsM NaCl and side B is fillec with 10 mOsM NaCl. Electrodes from a voltmeter are placed in each compartment and side A is set as ground (0 mV).
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
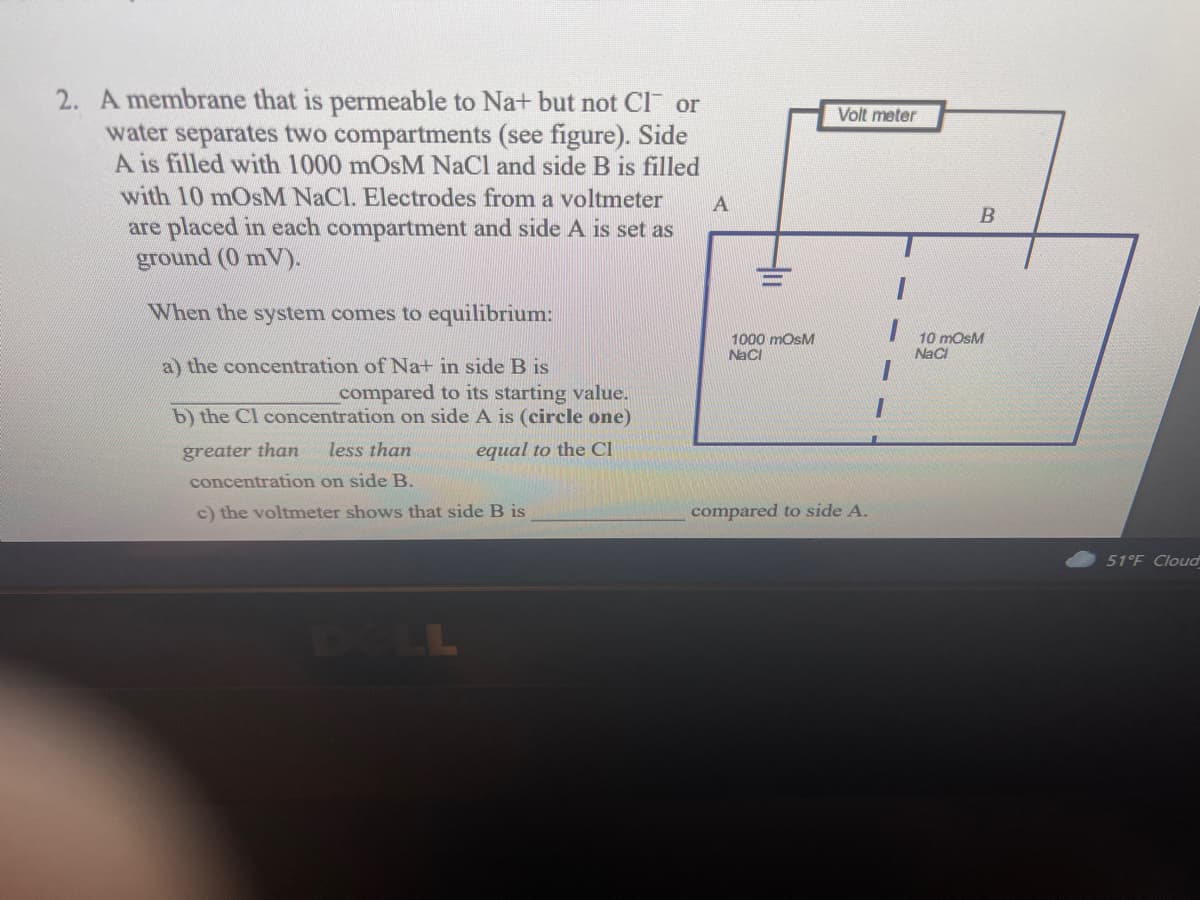
Transcribed Image Text:2. A membrane that is permeable to Na+ but not Cl or
Volt meter
water separates two compartments (see figure). Side
A is filled with 1000 mOsM NaCl and side B is filled
with 10 mOsM NaCl. Electrodes from a voltmeter
B
are placed in each compartment and side A is set as
ground (0 mV).
When the system comes to equilibrium:
10 mosM
Naci
1000 mOsM
NaCI
a) the concentration of Na+ in side B is
compared to its starting value.
b) the Cl concentration on side A is (circle one)
greater than
less than
equal to the Cl
concentration on side B.
c) the voltmeter shows that side B is
compared to side A.
51°F Cloud
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
