Q: 6. Predict the products of the following chemical reactions. -write the chemical formula of each…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: What is the role of phosphoric acid in the synthesis of the acetylsalicylic acid. please explain…
A: Phosphoric acid is a tribasic acid having three replaceable hydrogens Chemical formula of…
Q: 1. Provide the products for each polymerization shown below. Provide end groups where applicable. C.…
A: “Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Q: Predict the products of the following chemical reactions. -write the chemical formula of each…
A: #8: Ag(s) + HCl(aq): Ag(s) lies below H+(aq) in the electrochemical series. Hence Ag(s) is less…
Q: What is the difference between the mass number and the average atomic mass? Which one is on the…
A: we need to compare the mass number and the average atomic mass and tell which one of them is…
Q: Current Attempt in Progress The reagent needed (write the formula) to complete the following…
A:
Q: Write a mechanism for the step shown below, using curved arrows to show electron redistribution.…
A: Solution: Nucleophilic addition reaction is mainly monitored by attacking nucleophile, If there is…
Q: What is the name of this compound, NH4ClO3 2. What is the name of this compound, FeO 3. What is…
A: These can be given as:
Q: calculate the temperature (in K) of a 4.3L sample of gas in a balloon that is heated until it has…
A: For a definite amount of gas, at constant pressure, the volume of the gas increases with an increase…
Q: A gas mixture is made by combining 8.8 g8.8 g each of Ar,Ar, Ne,Ne, and an unknown diatomic gas. At…
A: 6
Q: How many grams of iron(II) carbonate, FeCO3 (formula weight = 115.8 g/mol), are precipitated when…
A:
Q: Name the hybridization of an inner atom that has 4 ligands and 2 lone pairs. Enter superscripts in…
A: Given -> 4 ligand , 2 loan pair Steric number = number of bond pair + number of loan pair…
Q: A simplified version of the computed energy profile for the Wittig reaction of a stabilised ylide…
A:
Q: 5. Reaction Enthalpy. balan and calculate AH for the following chemical equations. AHe (kJ/mol):…
A:
Q: What is the hydronium-ion concentration of a 3.95 x 10 M solution of p-bromobenzoic acid, BrCg…
A:
Q: Le Chatelier's Principle and Equilibrium Shifts 1. If the reaction below is initially at…
A: Le Chatelier's principle: According to the Le Chatelier's principle when a change in concentration…
Q: Calculate the density of HCN a highly poisonous gas at 250 degrees celsius and 2.00 atm pressure.
A:
Q: What is the pH of a solution with a hydrogen ion concentration of 6.55 X 10-6 M? Your Answer: Answer
A:
Q: For the following reaction step, indicate which pattern of arrow pushing it represents. OH H proton…
A:
Q: Write the word equation of the following reactions and identify which type of reaction has occurred.…
A: Single-displacement reaction: It is a chemical reaction in which one element is replaced by another…
Q: o A 55.0 mL solution of 0.150 M potassium alaninate ( H₂NC₂H₂CO₂K) is titrated with 0.150 M HCl. The…
A:
Q: In the hydrogen atom, what is the total number of nodes present in a 6d orbital? total nodes: How…
A:
Q: For the reaction: 2 Cu(s) + 1/2O2(g) → Cu₂O(s) AG° = -141 kJ/mol. What is the free energy change…
A: we need to calculate the free energy change for the reaction
Q: Cf+B4n+? Express your answer as a single isotope. Part B ΑΣΦ chemical reaction does not occur for…
A:
Q: At a certain temperature, 0.860 mol SO3 is placed in a 4.50 L container.…
A: Given : equilibrium reaction
Q: 4. A hot piece of metal (at 155 °C) with a mass of 4.68 g is placed into 53.9 grams of water at 22…
A: A question based on chemical thermodynamics that is to be accomplished.
Q: For the reaction COCl2g(?) ↔ ?o (?) + ?l2 2 (?), Kp was experimentally determined to be 0.0444 at…
A: Answer: In the given problem statement decomposition of COCl2 in CO and Cl2 is taking place and we…
Q: Question 8: What is the main organic product of the reaction in Figure 8? [Give a SMILES notation.]…
A:
Q: Please outline a synthesis for the following compound under the given restrictions and any other…
A:
Q: Which of the following is the most likely structure of the following cation after rearrangement? | B…
A:
Q: 1. Predict the product and state if it is thermodynamic or controlled x Br Br HBr -20°C HBr 40°C…
A:
Q: Is this reaction spontaneous? Why or why not? 1) S2O8^2- + Pb → 2SO4^2- + Pb^2+
A: The given reaction is a redox reaction. A spontaneous redox reaction has a positive cell potential.
Q: A 5.00 L tank at 5.15 °C is filled with 11.1 g of sulfur hexafluoride gas and 9.29 g of boron…
A:
Q: 1. Calculate the higher heating value in kj/kg of liquid dodecane fuel. The chemical formula of…
A: Given : The chemical formula of dodecane is C12H26
Q: Determine the energy (in J) associated with an electron being excited from n = 2 to n = 7. Give your…
A:
Q: To identify the result of the reaction between (R)-3-chloro-2,3-dimethylpentane and CH3CH2CH2OH at…
A: Given that Consider the reaction between (R)-3-chloro-2,3-dimethylpentane and CH3CH2CH2OH at room…
Q: For the equilibrium 21Br(g) 12(g) + Brz (9) K₂=8.5 x 10³ at 150 °C. if 2.8x102 atm of IBr is placed…
A: we need to calculate the equilibrium partial pressure of the species in the reaction
Q: Each of the following reactions is allowed to come to equilibrium and then the volume is changed as…
A: We would use Le-Chatelier's principle to determine the effect on changing the volume. According to…
Q: Propose a brief and efficient synthesis of trans-1,2-dimethylcyclohexane, starting with…
A: Using only Trans-2-butene as only source of carbon, we can use its 2 moles as shown below to form…
Q: do not fully undsertand the slide,need further explanation. what does it means by the additive…
A: The directing effect of groups attached to a benzene ring are of two types which are: (i):…
Q: onsider the reaction of 4,4-dipropylcyclohexan-1-ol with HI (hydrogen iodide). 3] Using curved…
A: The following reaction is a dehydration reaction of alcohol with an acid. The answer is as follows:…
Q: 23. The hydrogen ion concentration [H] of a lake in northern Ontario is 3.5 x 10 moles per litre.…
A: Since you have ask multiple questions, according to guidelines I am solving first one,if you want…
Q: Draw the major product of the substitution reaction shown below. Ignore any inorganic byproducts.
A:
Q: ______ times that of Xe(g)Xe(g) under the same conditions.
A: Effusion is a process in which a gas is allowed to escape under pressure through a fine or a small…
Q: Identify the correct arrow pushing: + CH₂- -CH=CH-CH₂ CH₂CH=CH-CH₂ CH₂CH=CH-CH₂ CH₂-CH=CH-CH₂…
A: While writing resonance stracture of any compound flow of electron always takes place from higher…
Q: 2H₂S (g) 2H2 (g) + S2 (g) was permitted to proceed to equilibrium. Contents of the 2-L reaction…
A:
Q: A sample of 10.9 g of Fe₂O, reacts with 16.8 g CO to yield Fe and CO₂. The balanced chemical…
A: Given -> Weight of Fe2O3 = 10.9 gm Weight of CO = 16.8 gm Fe2O3(s) +3 CO(g) ---> 2Fe(s) +…
Q: Identify the reactant or reactiants of the following reaction. CH3 O CH3 www CH3 "CH3
A:
Q: Calculate the OH concentration and pH of a 3.6 x 10-³ M aqueous solution of sodium cyanide, NaCN,…
A: According to the question we have, The concentration of the NaCN aqueous solution is given by (C) =…
Q: 1. Stomach acid (gastric acid) What is the source of H+ for stomach acid? 2. Acetylcholine →→→→…
A: The answer are as follows:
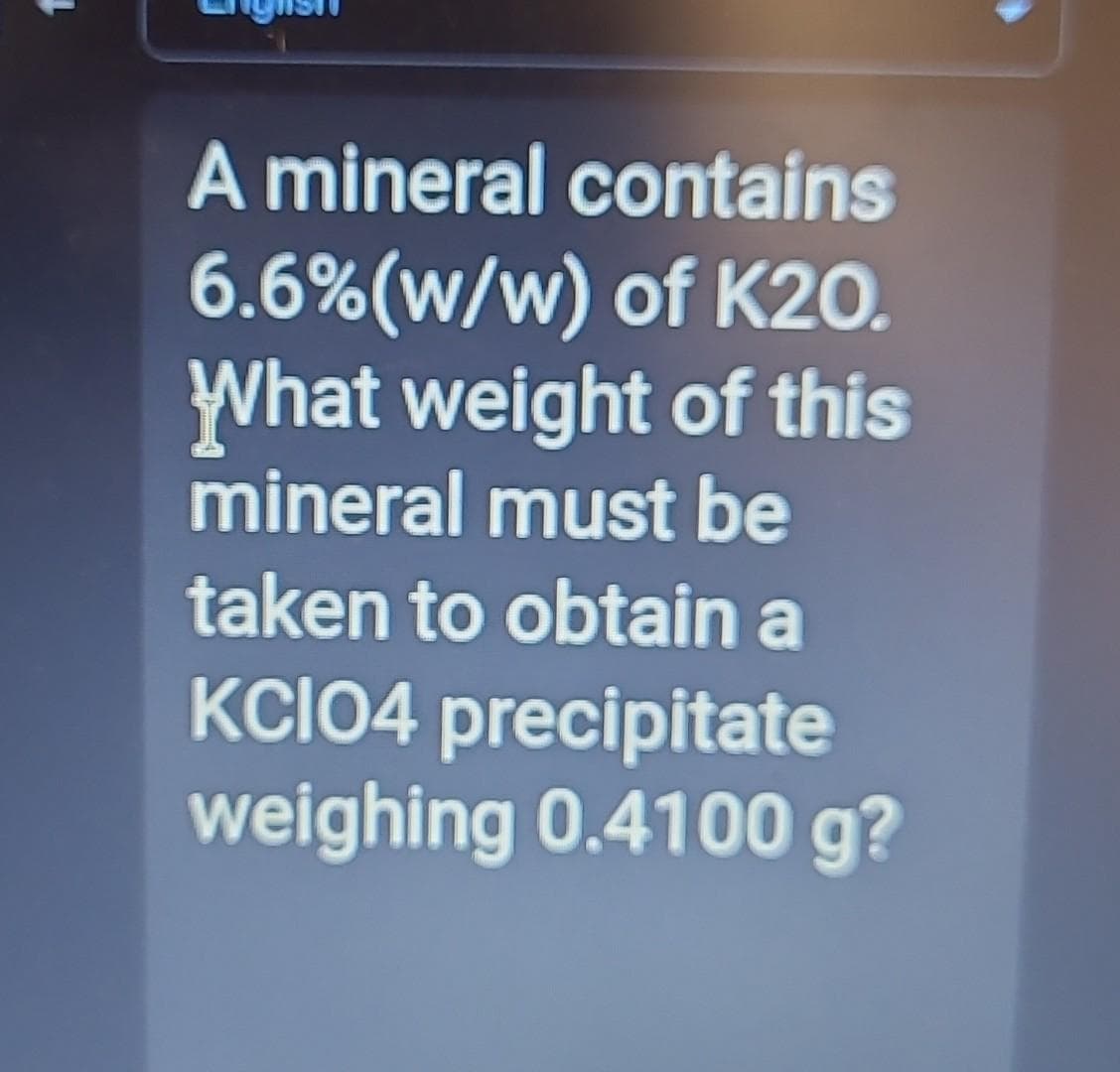
Step by step
Solved in 3 steps

- 31. A sample of meat scrap weighing 2.000 g is digested with concentrated H2SO4 and a catalyat. The resulting solution is made alkaline with NaOH and the liberated ammonia distilled into a 50.0 mL of 0.6700 N H2SO4. The excess then requires 30.10 mL of 0.06520 N NaOH for neutralization. What is the percentage of nitrogen in the meat?Ligand A binds to protein P with a dissociation constant, Kd = 1.5 uM.(a) What concentration of A is required in order for half of P to be bound?Use the equation: [PA]/ PTot = [A]/ Kd+[A] b)What concentration of A is required for 90% of P to be bound?A solution of sodium thiosulfate, Na2S2O3 was standardized by dissolving 0.188 g of KIO3 (MM = 214.00) in water, adding a large excess of KI, and acidifying with HCl. The liberated iodine required 18.25 mL of the thiosulfate solution to decolorize the blue starch/iodine complex. Calculate the molarity of Na2S2O3 solution. Stoichiometry of the reaction is: 1 mol IO3- = 3 mol I2 = 6 mol S2O32-
- Calculate the Ksp for Li3PO4 if at equilibrium the concentration of PO43− ion is 3.30 × 10−3 M.Cadmium is an interferant because it forms a precipitate with o-phenanthroline. What effect would the formation of a Cd-o-phenanthroline precipitate have on thedetermination of the parts per million of Fe in a sample?A solution of sodium thiosulfate was standardized by dissolving 0.1210 g KIO3 (MM=214) in water, adding a large excess of KI, and acidifying with HCI. The liberated iodine required 41.64 mL of the thiosulfate to decolorize the starch/iodine complex. Calculate the molarity of the Na₂S₂O3. 0.0721 M 0.239 M 0.1234 M 0.0815 M
- Protein X binds to Ligand W with a dissociation constant of .15 μmol. what is the concentration of W 75% of the protein is free (ie, unbound)?"Give out preparations of arena n6 complexesIf the concentration of PB2+, is found to be 1.3*10^-9mol/L in a saturated solution of Pb3(PO4)2, what is the ksp of Pb3(PO4)2?
- draw the electronic configuration of platinium II in carboplatine (complex and expect the followings: a. the charge of this drug b. the shape of its complex. c. the expected type of hybridization d. the number of coordination covalent bonds in this drug e. what is the pharmacological use of itWhat is the reaction resulting from the addition of SnCl2(aq) to a solution containing Bi3+ in a basic solution?Given A, B, and C… [A] U(VI) as uraninite; UO2 (where Fe2+= reductant; Fe(OH)3 ferrihydrite= product): 2Fe2+ + UO22+ +3H2O + H+ ---- > 2Fe(OH)3 + U4+ +2H2O [B] U(VI) as uraninite; UO2 (where Mn2+= reductant; MnO2 pyrolusite= product): 2H2O + UO22+ + Mn2+ ---- > UO2 + B - MnO2 + 4H+ [C] U(VI) as as uraninite; UO2 (where HS-= reductant; S0= product): UO22+ + Hs- ---- > UO2 + S + H+ QUESTION: Use thermodynamic calculations [use redox potential (Eh)] to predict which of the three reductants below is most favorable at pH 3 ? (1) Fe2+ (2) Mn2+ (3) HS-

