A mixture of A and B has a liquid-vapor phase diagram as shown in the picture which is not ideal. Assume that 1.5 mol of A and 3.5 mol of B were mixed in an isothermal vessel. Then equilibration was done, and the total vapor pressure was determined to be 50.0 torr. Solve for the number of moles of B that is present in the vapor phase. Choose your answer. a. 1.43 mol b. 1.07 mol c. 1.82 mol d. 2.43 mol
A mixture of A and B has a liquid-vapor phase diagram as shown in the picture which is not ideal. Assume that 1.5 mol of A and 3.5 mol of B were mixed in an isothermal vessel. Then equilibration was done, and the total vapor pressure was determined to be 50.0 torr. Solve for the number of moles of B that is present in the vapor phase. Choose your answer. a. 1.43 mol b. 1.07 mol c. 1.82 mol d. 2.43 mol
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.17QAP
Related questions
Question
A mixture of A and B has a liquid-vapor phase diagram as shown in the picture which is not ideal. Assume that 1.5 mol of A and 3.5 mol of B were mixed in an isothermal vessel. Then equilibration was done, and the total vapor pressure was determined to be 50.0 torr. Solve for the number of moles of B that is present in the vapor phase. Choose your answer.
a. 1.43 mol
b. 1.07 mol
c. 1.82 mol
d. 2.43 mol
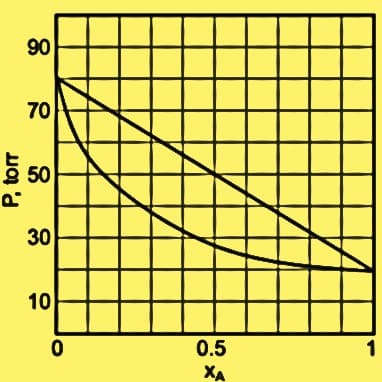
Transcribed Image Text:06
70
5 50
P.
30
10
0.5
XA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning