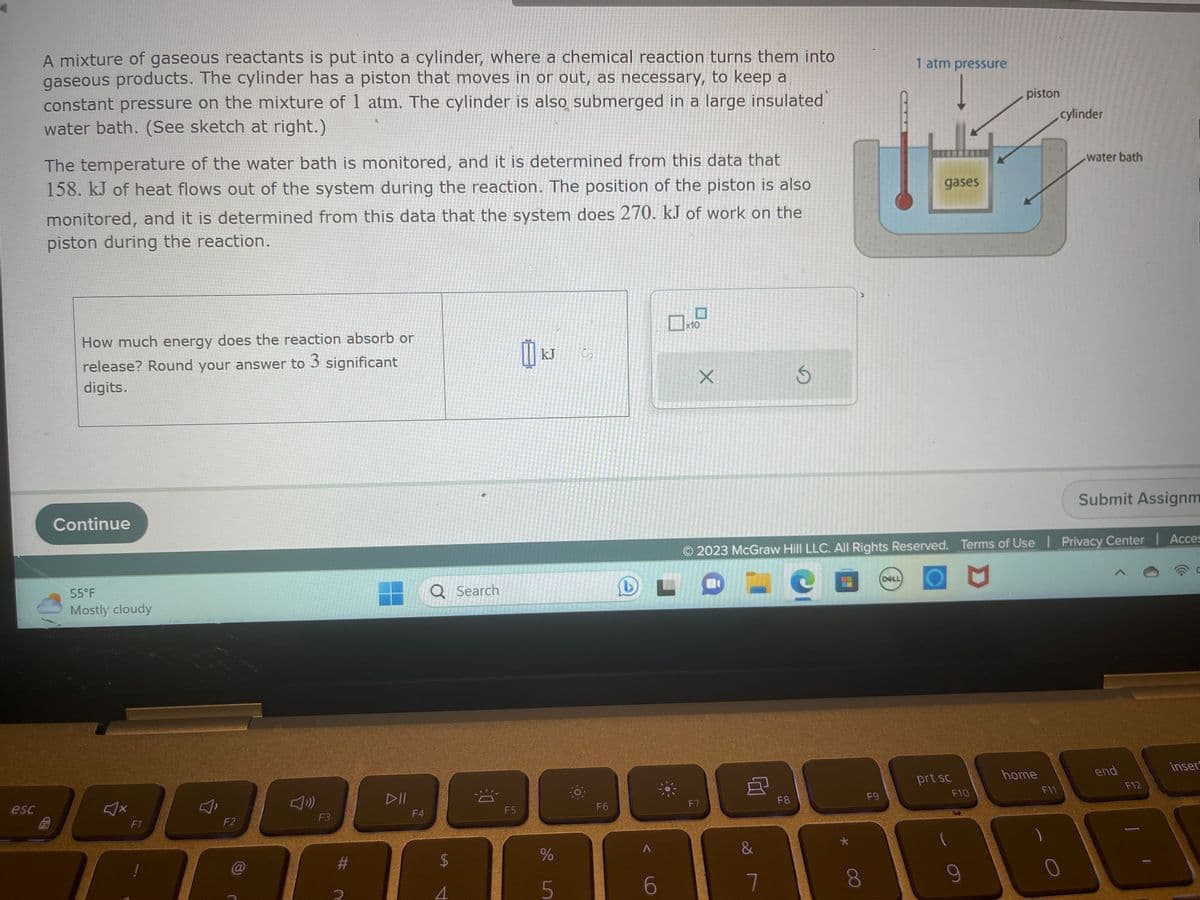
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) The temperature of the water bath is monitored, and it is determined from this data that 158. kJ of heat flows out of the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the system does 270. kJ of work on the piston during the reaction. How much energy does the reaction absorb or release? Round your answer to 3 significant digits. Continue 55°F Mostly cloudy - Q Search b X S 1 atm pressure mitum gases piston cylinder water bath Submit Assignm Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) The temperature of the water bath is monitored, and it is determined from this data that 158. kJ of heat flows out of the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the system does 270. kJ of work on the piston during the reaction. How much energy does the reaction absorb or release? Round your answer to 3 significant digits. Continue 55°F Mostly cloudy - Q Search b X S 1 atm pressure mitum gases piston cylinder water bath Submit Assignm Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.32E: Many compressed gases come in large,heavy metal cylindersthat are so heavy that they need a special...
Related questions
Question
Transcribed Image Text:esc
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into
gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a
constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated
water bath. (See sketch at right.)
The temperature of the water bath is monitored, and it is determined from this data that
158. kJ of heat flows out of the system during the reaction. The position of the piston is also
monitored, and it is determined from this data that the system does 270. kJ of work on the
piston during the reaction.
3D
Fo
How much energy does the reaction absorb or
release? Round your answer to 3 significant
digits.
Continue
55°F
Mostly cloudy
F1
F2
@
F3
PEN
#
F4
Q Search
LA
4
12
F5
kJ
%
5
S
F6
A
6
x10
X
F7
&
7
S
F8
F9
1 atm pressure
2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces
DELL
gases
prt sc
F10
piston
9
home
cylinder
F11
water bath
Submit Assignm
end
F12
J
inser
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning