A model for H2O is shown in the chem3D window. H2O has bent geometry. This geometry is sometimes called angular or V-shaped geometry. ball & stick + labels Rotate the molecule until you have a feeling for its three-dimensional shape. How many atoms are bonded to the central atom? Consider the bond angle at the central atom. What is the approximate numerical value of this angle? v degrees. You could view this geometry as being derived from a trigonal planar molecule by removing one atom, or as being derived from a tetrahedral molec by removing two atoms. Which does the bond angle suggest? | For practice, type in the name of the geometry of the molecule:
A model for H2O is shown in the chem3D window. H2O has bent geometry. This geometry is sometimes called angular or V-shaped geometry. ball & stick + labels Rotate the molecule until you have a feeling for its three-dimensional shape. How many atoms are bonded to the central atom? Consider the bond angle at the central atom. What is the approximate numerical value of this angle? v degrees. You could view this geometry as being derived from a trigonal planar molecule by removing one atom, or as being derived from a tetrahedral molec by removing two atoms. Which does the bond angle suggest? | For practice, type in the name of the geometry of the molecule:
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter7: Chemical Bonding And Molecular Geometry
Section: Chapter Questions
Problem 114E: Use the Molecule Shape simulator (http://openstaxcollege.org/I/6MolecShape) to build a molecule....
Related questions
Question
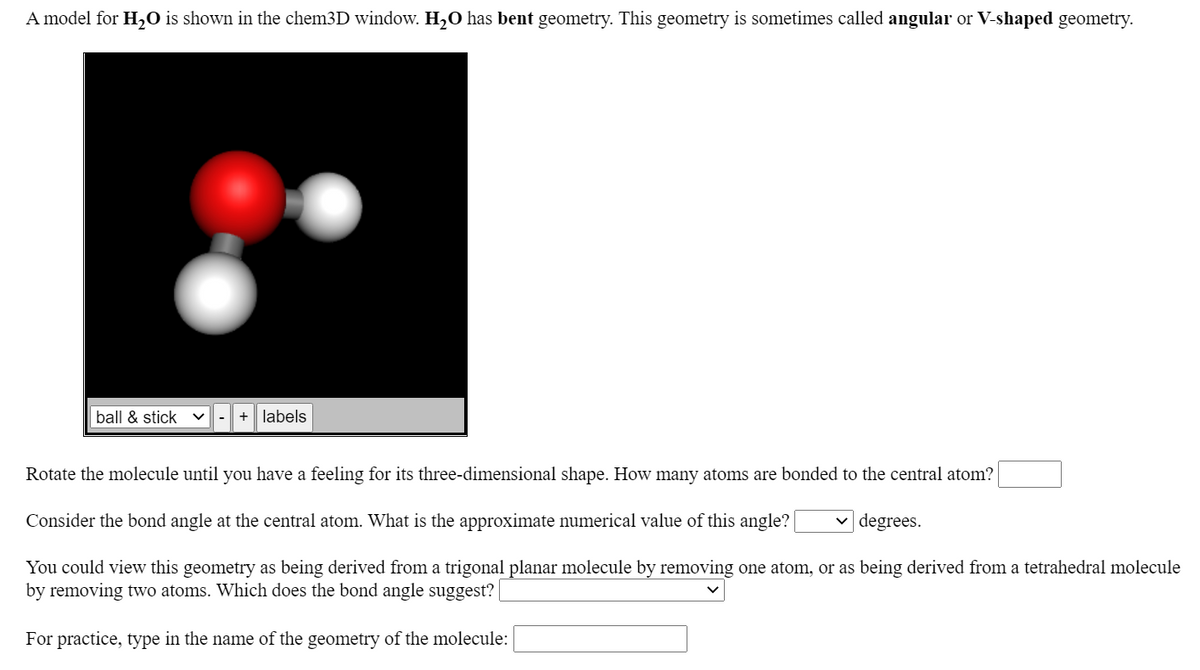
Transcribed Image Text:A model for H,O is shown in the chem3D window. H2O has bent geometry. This geometry is sometimes called angular or V-shaped geometry.
ball & stick
+| labels
-
Rotate the molecule until you have a feeling for its three-dimensional shape. How many atoms are bonded to the central atom?
Consider the bond angle at the central atom. What is the approximate numerical value of this angle?
degrees.
You could view this geometry as being derived from a trigonal planar molecule by removing one atom, or as being derived from a tetrahedral molecule
by removing two atoms. Which does the bond angle suggest?
For practice, type in the name of the geometry of the molecule:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning