A piece of metal weighing 83.60 g was heated to 99.6°C and then put into 41.90 g of water (initially at 16.2°C ). The metal and water were allowed to come to the same temperature, determined to be 27.3°C . Assuming no heat lost to the environment, calculate the specific heat of the metal. (CH2O = 4.181 J/g°C) %3D Round your answer to 2 decimal places. Your Answer:
A piece of metal weighing 83.60 g was heated to 99.6°C and then put into 41.90 g of water (initially at 16.2°C ). The metal and water were allowed to come to the same temperature, determined to be 27.3°C . Assuming no heat lost to the environment, calculate the specific heat of the metal. (CH2O = 4.181 J/g°C) %3D Round your answer to 2 decimal places. Your Answer:
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 12E: An aluminum kettle weighs 1.05 kg. (a) What is the heat capacity of the kettle? (b) How much heat is...
Related questions
Question
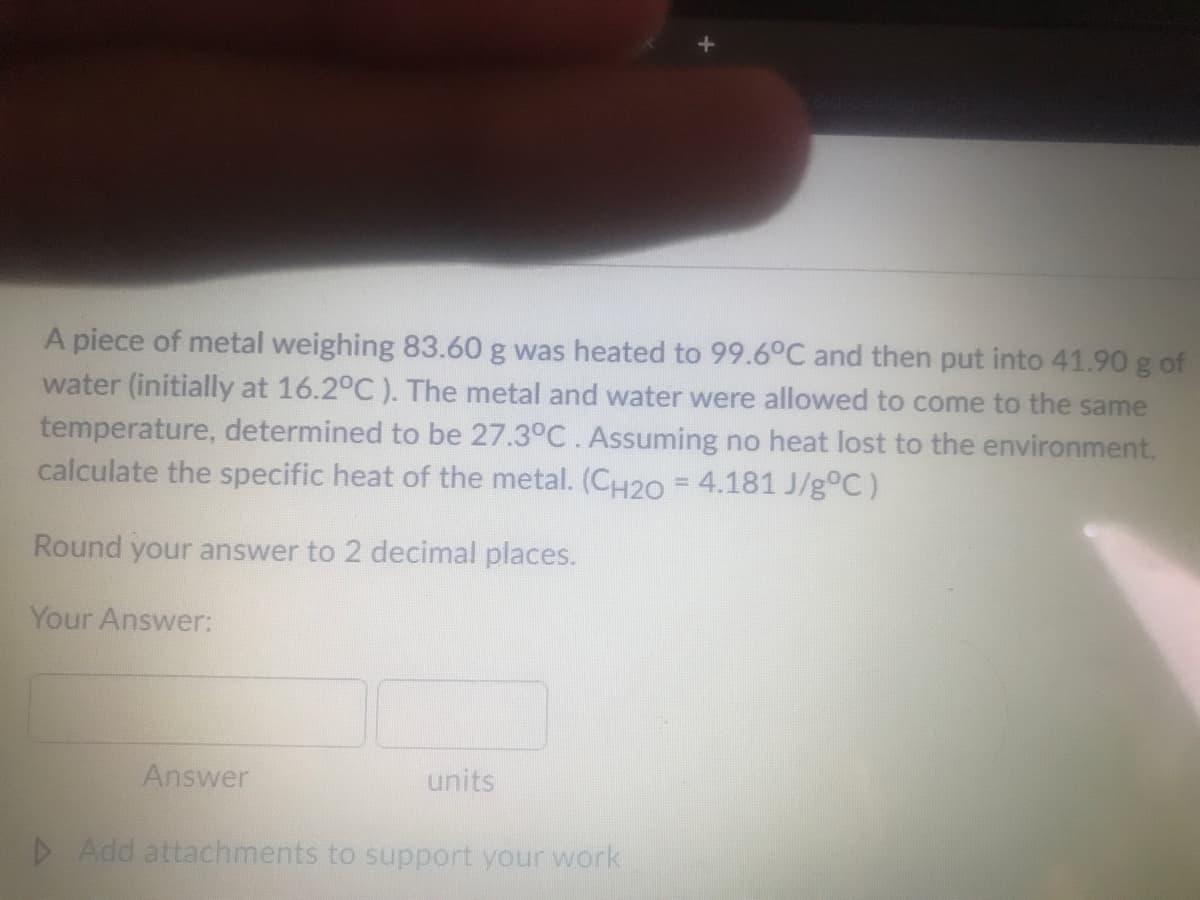
Transcribed Image Text:A piece of metal weighing 83.60 g was heated to 99.6°C and then put into 41.90 g of
water (initially at 16.2°C ). The metal and water were allowed to come to the same
temperature, determined to be 27.3°C. Assuming no heat lost to the environment,
calculate the specific heat of the metal. (CH2O = 4.181 J/g°C)
%3D
Round your answer to 2 decimal places.
Your Answer:
Answer
units
D Add attachments to support your work
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning