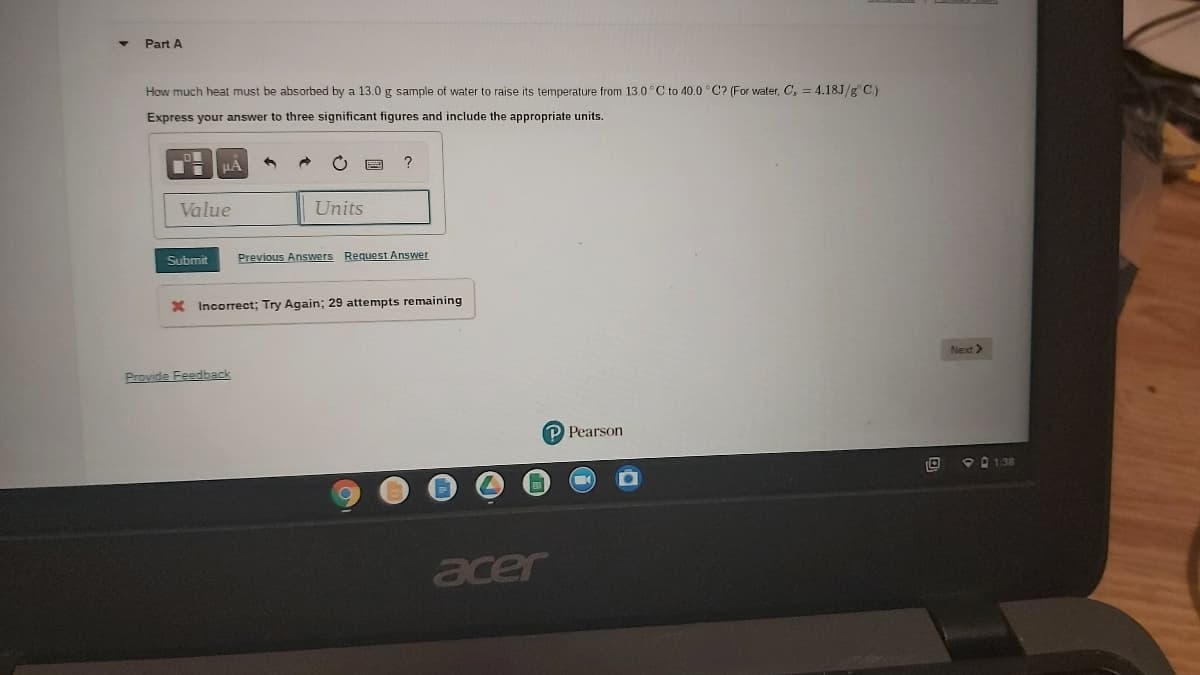
How much heat must be absorbed by a 13.0 g sample of water to raise its temperature from 13.0°C to 40.0 °C? (For water, C, = 4.18J/g C) Express your answer to three significant figures and include the appropriate units.
How much heat must be absorbed by a 13.0 g sample of water to raise its temperature from 13.0°C to 40.0 °C? (For water, C, = 4.18J/g C) Express your answer to three significant figures and include the appropriate units.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.2: Specific Heat Capacity: Heating And Cooling
Problem 5.2CYU: A 15.5-g piece of chromium, heated to 100.0 C, is dropped into 55.5 g of water at 16.5 C. The final...
Related questions
Question
100%
Transcribed Image Text:Part A
How much heat must be absorbed by a 13.0 g sample of water to raise its temperature from 13.0°C to 40.0 °C? (For water, C, = 4.18J/g C)
Express your answer to three significant figures and include the appropriate units.
HA
Value
Units
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 29 attempts remaining
Next>
Provide Feedback
P Pearson
P0 1.38
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning