A potentiometric titration of a 0.278 g sample of FeSO4.7H₂O salt, in acidic medium, with a 0.011 M solution of potassium dichromate (K,Cr:O) was conducted. Cr₂0 + 6 Fe + 14 H → 2Cr + 6 Fe" + 7 H₂O The potential is measured with respect to a calomel electrode (E-246 mV). Graph 1 represents the measured potential with respect to the volume of added dichromate. Calculate E for the half-reaction: Fe + e-Fe (Show your work using Nernst equation). Hint: Use the graph, to determine, approximately, the equivalence point and mid-point of the titration
A potentiometric titration of a 0.278 g sample of FeSO4.7H₂O salt, in acidic medium, with a 0.011 M solution of potassium dichromate (K,Cr:O) was conducted. Cr₂0 + 6 Fe + 14 H → 2Cr + 6 Fe" + 7 H₂O The potential is measured with respect to a calomel electrode (E-246 mV). Graph 1 represents the measured potential with respect to the volume of added dichromate. Calculate E for the half-reaction: Fe + e-Fe (Show your work using Nernst equation). Hint: Use the graph, to determine, approximately, the equivalence point and mid-point of the titration
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.12QAP
Related questions
Question
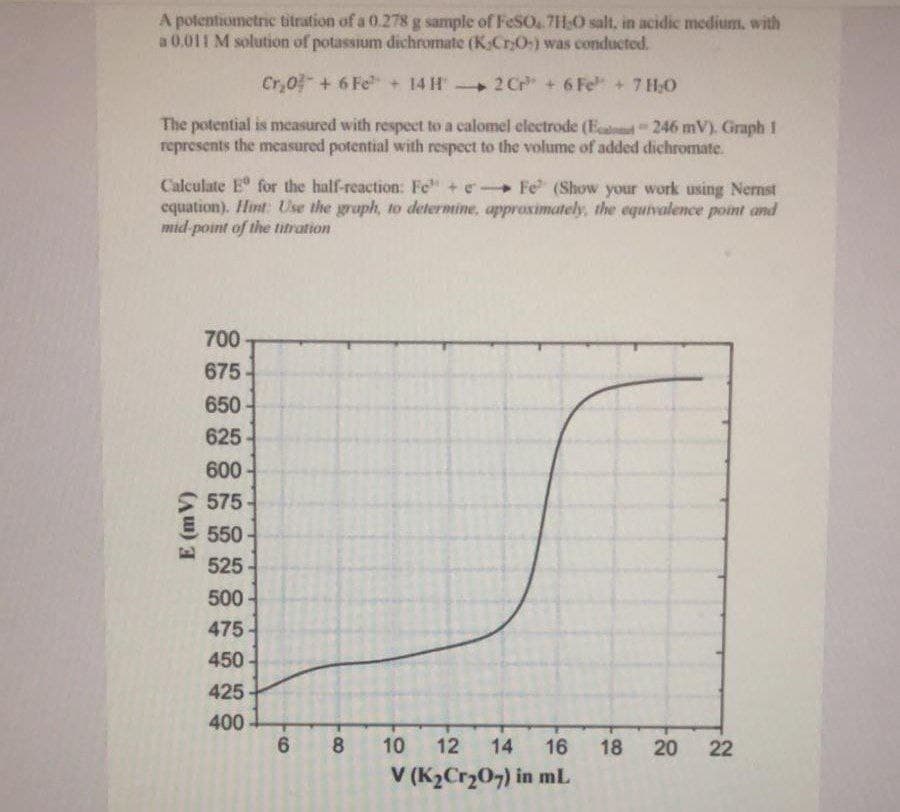
Transcribed Image Text:A potentiometric titration of a 0.278 g sample of FeSO4.7H,O salt, in acidic medium, with
a 0.011 M solution of potassium dichromate (K.Cr₂O) was conducted.
Cr₂0 + 6 Fe + 14 H →2Cr + 6 Fe" + 7 H₂O
The potential is measured with respect to a calomel electrode (E246 mV). Graph 1
represents the measured potential with respect to the volume of added dichromate.
Calculate E for the half-reaction: Fe + e-Fe (Show your work using Nernst
equation). Hint: Use the graph, to determine, approximately, the equivalence point and
mid-point of the titration
700
675
650
625
600
575
550
525
500
475
450
425
400
18 20
22
10 12 14 16
V (K₂Cr₂O7) in mL
E (MV)
6 8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning