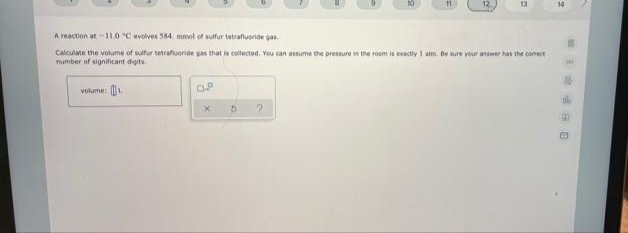
A reaction at-11.0 C evolves S84. mmel of sutur tetraftuoride gas Calculate the volume of sutur tetrauoride as that is collected. You can aume the pressure in the room is eacty I m. ee your answer has the camectr number of significant digits volume e RO
A reaction at-11.0 C evolves S84. mmel of sutur tetraftuoride gas Calculate the volume of sutur tetrauoride as that is collected. You can aume the pressure in the room is eacty I m. ee your answer has the camectr number of significant digits volume e RO
Chapter5: Gases
Section: Chapter Questions
Problem 61E: An ideal gas is contained in a cylinder with a volume of 5.0 102 mL at a temperature of 30.C and a...
Related questions
Question
Solve it please...
Transcribed Image Text:13
14
A reaction at-11.0 °C evolves 584. mol of sulfur tetrafluoride gas
Calculate the volume of sulfur tetrafuoride pas that is collected. You can assume the pressure in the room is exactiy I atm. Be sure your answer has the comect
number of significant digits
volume:
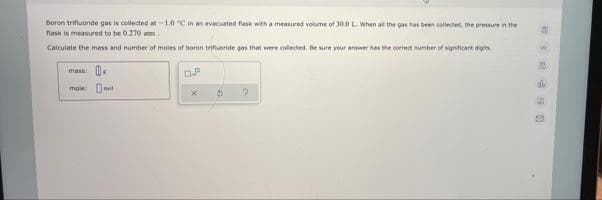
Transcribed Image Text:Boron trifiuoride gas is collected at-1.0 C in an evacuated flask with a measured volume of 30.0 L When al the gas ha been collecte, the pressure in the
nask is measured to be 0.270 ann.
Calculate the imass and number of moles of boron trifueride gas that were colected, Be sure your anwer has the correct umber of signficant dgts
mass: .
mole: O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax