A recent article reported that benzylic bromination can be achieved photochemically using bromotrichloromethane as the brominating agent (you used NBS in lab) as shown below. Draw the mechanism of this reaction, labeling all sets as either "Initiation" or "Propagation". Also, show one feasible termination step. Helpful BDE's are given. How many unique hydrogens (NMR) are present in the product? Br Org. Biomol. Chem. 2019, 17, 1384 BrCCl3 hv Мео OMe Мео OMe OMe ОMe BDE of C-Br bond ~230 kJ/mol BDE of C-H in HCClg~ 401 kJ/mol
A recent article reported that benzylic bromination can be achieved photochemically using bromotrichloromethane as the brominating agent (you used NBS in lab) as shown below. Draw the mechanism of this reaction, labeling all sets as either "Initiation" or "Propagation". Also, show one feasible termination step. Helpful BDE's are given. How many unique hydrogens (NMR) are present in the product? Br Org. Biomol. Chem. 2019, 17, 1384 BrCCl3 hv Мео OMe Мео OMe OMe ОMe BDE of C-Br bond ~230 kJ/mol BDE of C-H in HCClg~ 401 kJ/mol
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 30CTQ
Related questions
Question
Could you help me complete this practice problem, I would like to check my work. Thank you for your time!
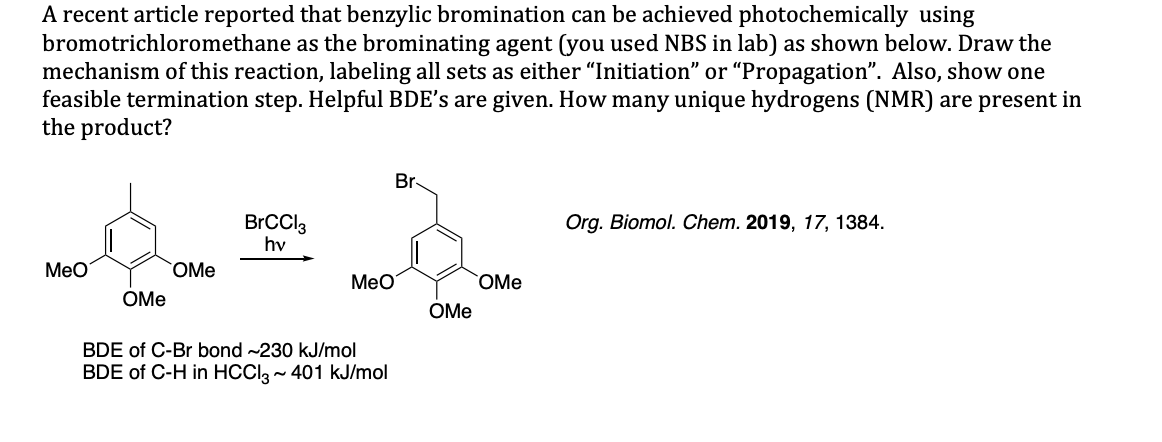
Transcribed Image Text:A recent article reported that benzylic bromination can be achieved photochemically using
bromotrichloromethane as the brominating agent (you used NBS in lab) as shown below. Draw the
mechanism of this reaction, labeling all sets as either "Initiation" or "Propagation". Also, show one
feasible termination step. Helpful BDE's are given. How many unique hydrogens (NMR) are present in
the product?
Br
Org. Biomol. Chem. 2019, 17, 1384
BrCCl3
hv
Мео
OMe
Мео
OMe
OMe
ОMe
BDE of C-Br bond ~230 kJ/mol
BDE of C-H in HCClg~ 401 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning