a) Sketch the molecular orbital energy level diagram for the heteronuclear diatomic molecule AB (not necessarily in scale), where A and B are two hypothetical atoms. Useful data: s-p mixing does not occur in AB. Electron configuration of A: (1s)² (2s)² (2p)² Atomic orbital energies of A: 1s = -396 eV, 2s = -25 eV, 2p = -12 eV Electron configuration of B: (1s)² (2s)² (2p) Atomic orbital energies of B: -530 eV, 2s = -32 eV, 2p = -20 eV
a) Sketch the molecular orbital energy level diagram for the heteronuclear diatomic molecule AB (not necessarily in scale), where A and B are two hypothetical atoms. Useful data: s-p mixing does not occur in AB. Electron configuration of A: (1s)² (2s)² (2p)² Atomic orbital energies of A: 1s = -396 eV, 2s = -25 eV, 2p = -12 eV Electron configuration of B: (1s)² (2s)² (2p) Atomic orbital energies of B: -530 eV, 2s = -32 eV, 2p = -20 eV
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 39P: The photoelectron spectrum of HBr has two main groups of peaks. The first has ionization energy...
Related questions
Question
help
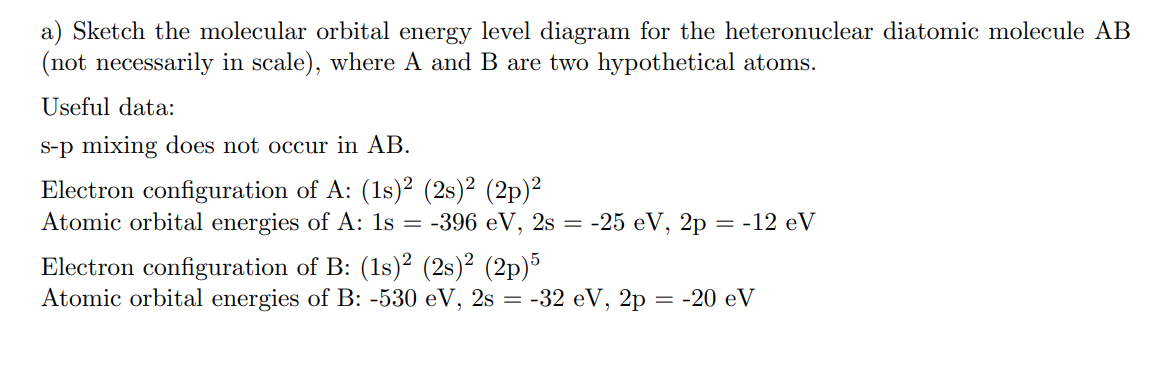
Transcribed Image Text:a) Sketch the molecular orbital energy level diagram for the heteronuclear diatomic molecule AB
(not necessarily in scale), where A and B are two hypothetical atoms.
Useful data:
s-p mixing does not occur in AB.
Electron configuration of A: (1s)² (2s)² (2p)²
Atomic orbital energies of A: 1s = -396 eV, 2s = -25 eV, 2p
= -12 eV
Electron configuration of B: (1s)² (2s)² (2p)%
Atomic orbital energies of B: -530 eV, 2s = -32 eV, 2p = -20 eV
Expert Solution
Step 1 Analysis

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER