1. Derive the following expression for the bonding and antibonding orbitals of a heteronuclear diatomic molecule consisting of two electrons in a molecular orbital of the form y = cAA + CBB with atomic orbitals A and B. Let aA=HAA, AB=HBB, B=HAB=HBA, SAA=SBB=1, and, as a simplification, let SAB=SBA=S=0 (zero-overlap approximation). Note that the assumption of SAB=SBA=S=0 is used simply to get a more transparent expression. Show that the secular determinant is given by a, - E = 0 - E and energies are given by 1/2 2B E̟ = }(a, +a,)±±(a,- For B<0, E. is the lower energy solution.
1. Derive the following expression for the bonding and antibonding orbitals of a heteronuclear diatomic molecule consisting of two electrons in a molecular orbital of the form y = cAA + CBB with atomic orbitals A and B. Let aA=HAA, AB=HBB, B=HAB=HBA, SAA=SBB=1, and, as a simplification, let SAB=SBA=S=0 (zero-overlap approximation). Note that the assumption of SAB=SBA=S=0 is used simply to get a more transparent expression. Show that the secular determinant is given by a, - E = 0 - E and energies are given by 1/2 2B E̟ = }(a, +a,)±±(a,- For B<0, E. is the lower energy solution.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 78E: The diatomic molecule OH exists in the gas phase. The bond length and bond energy have been measured...
Related questions
Question
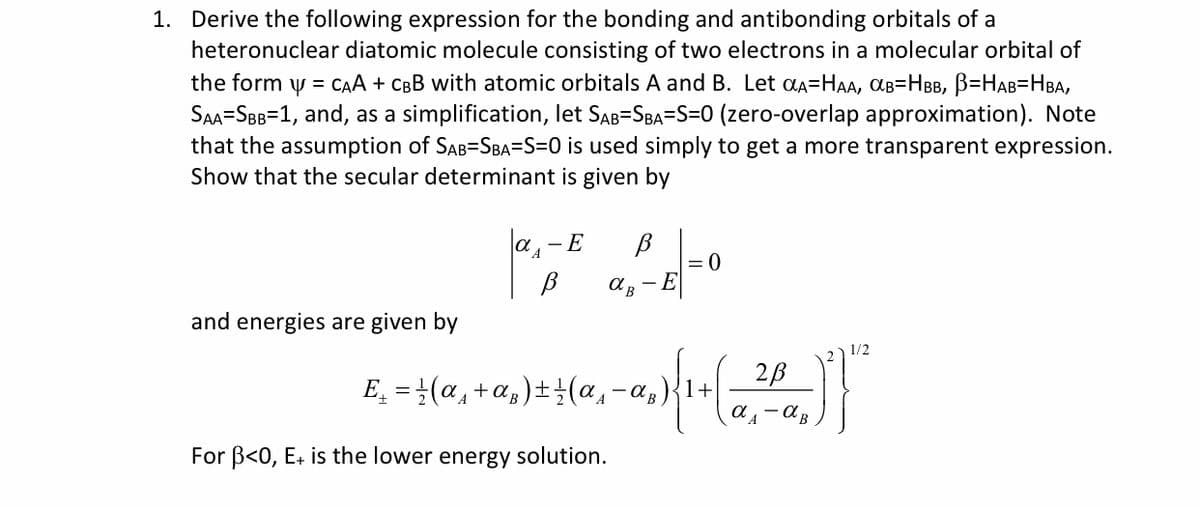
Transcribed Image Text:1. Derive the following expression for the bonding and antibonding orbitals of a
heteronuclear diatomic molecule consisting of two electrons in a molecular orbital of
the form y = CAA + CBB with atomic orbitals A and B. Let aa=HAA, aB=HBB, B=HAB=HBA,
SAA-SBB=1, and, as a simplification, let SAB=SBA=S=0 (zero-overlap approximation). Note
that the assumption of SAB=SBA=S=0 is used simply to get a more transparent expression.
Show that the secular determinant is given by
a,-E
- E
and energies are given by
1/2
2B
E̟ = }(a, +a,)±(a,-a,)
For B<0, E+ is the lower energy solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning