A small amount of acetonitrile (CH3CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketch below (picture 1) A. The enthalpy of solution (delta Hsoln) is negative when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. B. Would he be absorbed, released, or neither, if the system moved from stage A to B? C. What force would oppose or favor the system moving from stage a to B? Select all that apply. (Picture2)
A small amount of acetonitrile (CH3CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketch below (picture 1) A. The enthalpy of solution (delta Hsoln) is negative when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. B. Would he be absorbed, released, or neither, if the system moved from stage A to B? C. What force would oppose or favor the system moving from stage a to B? Select all that apply. (Picture2)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 5CR
Related questions
Question
A small amount of acetonitrile (CH3CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketch below (picture 1)
A. The enthalpy of solution (delta Hsoln) is negative when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy.
B. Would he be absorbed, released, or neither, if the system moved from stage A to B?
C. What force would oppose or favor the system moving from stage a to B? Select all that apply. (Picture2)
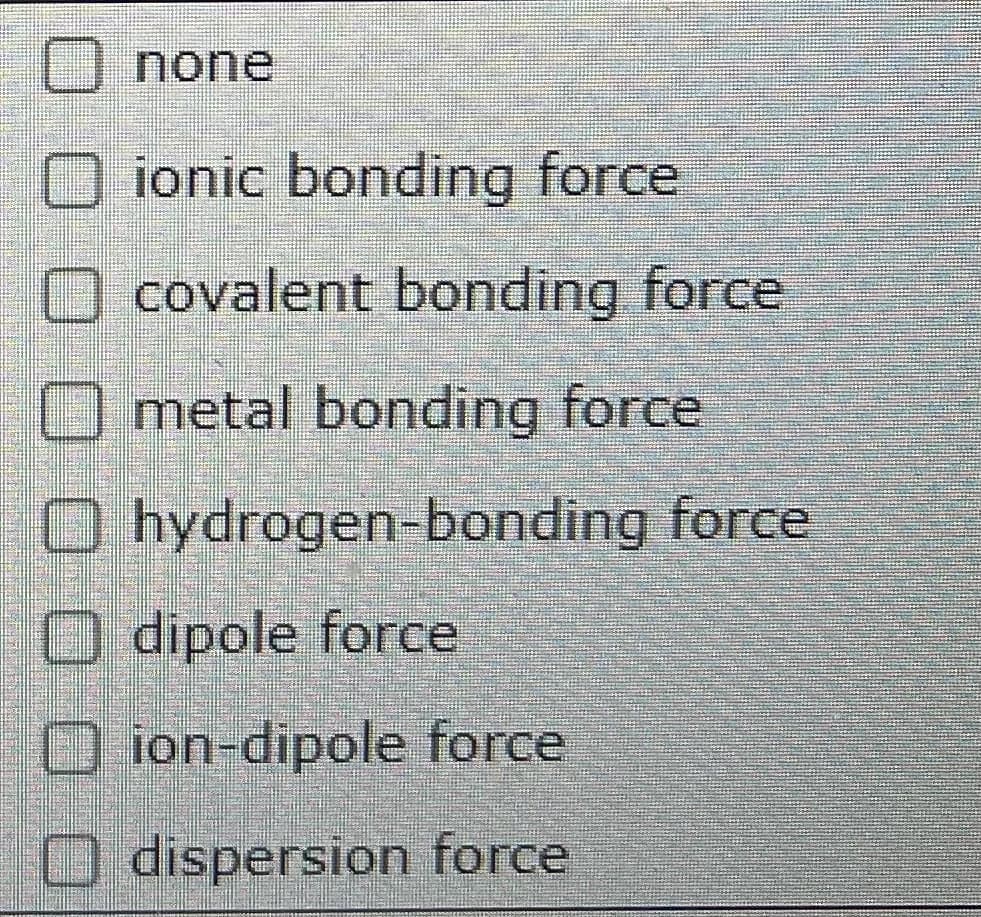
Transcribed Image Text:none
O ionic bonding force
O covalent bonding force
O metal bonding force
O hydrogen-bonding force
O dipole force
O ion-dipole force
O dispersion force
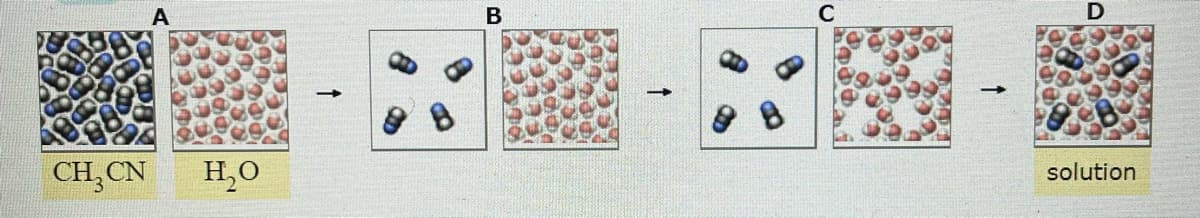
Transcribed Image Text:C
CH,CN
H,0
solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax