A so-called PBS buffer (phosphate buffered saline) is prepared by according to the given protocol: Prepare 800 mL of distilled water in a suitable container; Add 8 g of NaCl to the solution; Add 200 mg of KCl to the solution; Add 1.44 g of Na₂HPO4 to the solution; Add 245 mg of KH₂PO4 to the solution; Adjust solution to desired pH (typically pH ≈ 7.4); and Add distilled water until volume is 1 L. (a) Calculate the pH of the initial solution (before pH is adjusted). (b) How many moles of acid or base are required to adjust the pH to 7.4?
A so-called PBS buffer (phosphate buffered saline) is prepared by according to the given protocol: Prepare 800 mL of distilled water in a suitable container; Add 8 g of NaCl to the solution; Add 200 mg of KCl to the solution; Add 1.44 g of Na₂HPO4 to the solution; Add 245 mg of KH₂PO4 to the solution; Adjust solution to desired pH (typically pH ≈ 7.4); and Add distilled water until volume is 1 L. (a) Calculate the pH of the initial solution (before pH is adjusted). (b) How many moles of acid or base are required to adjust the pH to 7.4?
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 2ASA: In an acid-base titration, 21.16 mL of an NaOH solution are needed to neutralize 20.04 mL of a...
Related questions
Question
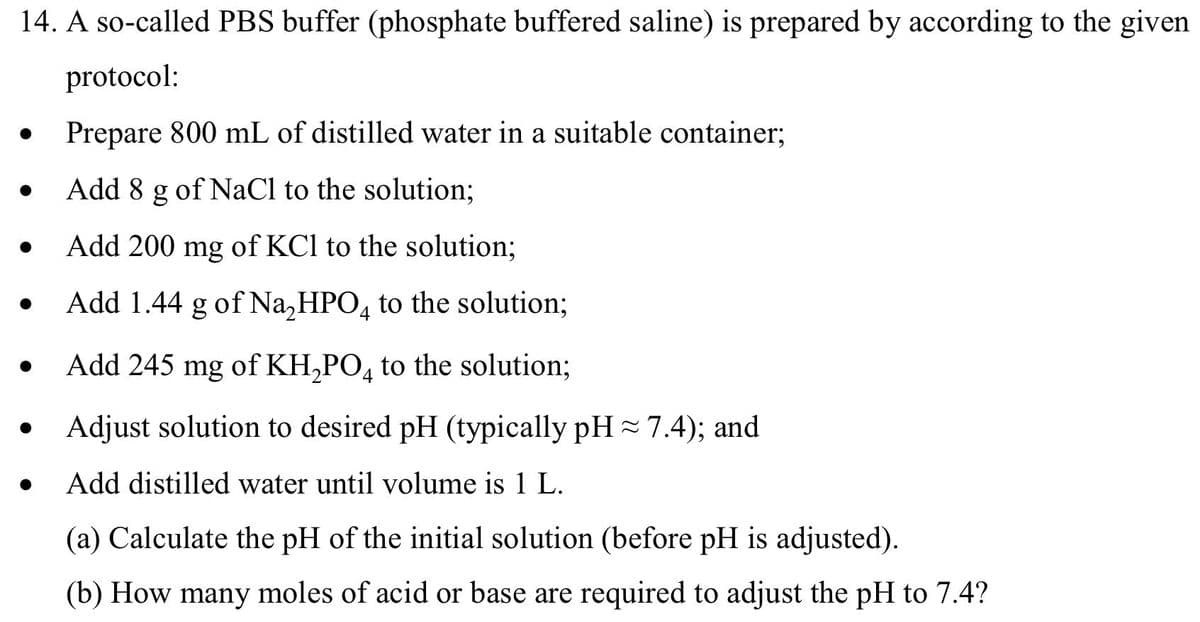
Transcribed Image Text:14. A so-called PBS buffer (phosphate buffered saline) is prepared by according to the given
protocol:
●
Prepare 800 mL of distilled water in a suitable container;
●
Add 8 g of NaCl to the solution;
Add 200 mg of KCl to the solution;
Add 1.44 g of Na₂HPO4 to the solution;
Add 245 mg of KH₂PO4 to the solution;
Adjust solution to desired pH (typically pH ≈ 7.4); and
Add distilled water until volume is 1 L.
(a) Calculate the pH of the initial solution (before pH is adjusted).
(b) How many moles of acid or base are required to adjust the pH to 7.4?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
