Label a curve of potential energy vs. dihedral angle for the Newman conformations of 2,3-dimethylbutane. In each case, the C-2 to C-3 bond rotates in the clockwise direction. Step 1: Translate line-bond notation to the Newman projection. Step 2: Identify Newman projections for each rotation. HI!!!! "Η Step 3: Identify the relative stability of each Newman projection. Step 4: Construct the energy diagram. Step 4: Construct the energy diagram. You have determined the different Newman projections and the relative stabilities. Plot a potential energy vs. dihedral angle graph for the Newman conformations of 2,3-dimethylbutane. Return to the previous questions to view the substituents at each angle of bond rotation and the relative stability. Points on the graphing module are graded for location. When you click and hold onto the first dot, highlighted points appear that represent possible positions for the dot. Consider the energy level for each angle of rotation, then drag the dot to the appropriate energy level and let go to drop. All points must be moved. Click and drag each point to the appropriate energy level for each Newman projection. Potential Energy 100 90 80 70 60 50 40 30 20 10 0 60 120 180 240 Angle of internal rotation (dihedral angle) 300 360
Label a curve of potential energy vs. dihedral angle for the Newman conformations of 2,3-dimethylbutane. In each case, the C-2 to C-3 bond rotates in the clockwise direction. Step 1: Translate line-bond notation to the Newman projection. Step 2: Identify Newman projections for each rotation. HI!!!! "Η Step 3: Identify the relative stability of each Newman projection. Step 4: Construct the energy diagram. Step 4: Construct the energy diagram. You have determined the different Newman projections and the relative stabilities. Plot a potential energy vs. dihedral angle graph for the Newman conformations of 2,3-dimethylbutane. Return to the previous questions to view the substituents at each angle of bond rotation and the relative stability. Points on the graphing module are graded for location. When you click and hold onto the first dot, highlighted points appear that represent possible positions for the dot. Consider the energy level for each angle of rotation, then drag the dot to the appropriate energy level and let go to drop. All points must be moved. Click and drag each point to the appropriate energy level for each Newman projection. Potential Energy 100 90 80 70 60 50 40 30 20 10 0 60 120 180 240 Angle of internal rotation (dihedral angle) 300 360
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter7: Cycloalkanes
Section: Chapter Questions
Problem 17E: Build a model of methylcyclohexane, and use the model to complete the following Newmanprojections of...
Related questions
Question
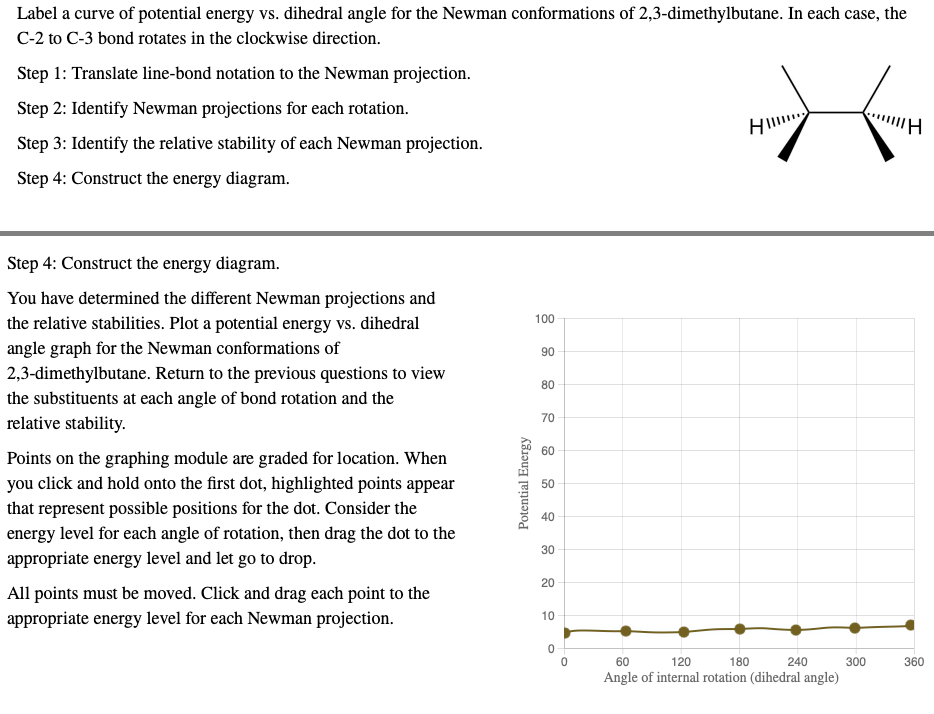
Transcribed Image Text:Label a curve of potential energy vs. dihedral angle for the Newman conformations of 2,3-dimethylbutane. In each case, the
C-2 to C-3 bond rotates in the clockwise direction.
Step 1: Translate line-bond notation to the Newman projection.
Step 2: Identify Newman projections for each rotation.
HI!!!!
"Η
Step 3: Identify the relative stability of each Newman projection.
Step 4: Construct the energy diagram.
Step 4: Construct the energy diagram.
You have determined the different Newman projections and
the relative stabilities. Plot a potential energy vs. dihedral
angle graph for the Newman conformations of
2,3-dimethylbutane. Return to the previous questions to view
the substituents at each angle of bond rotation and the
relative stability.
Points on the graphing module are graded for location. When
you click and hold onto the first dot, highlighted points appear
that represent possible positions for the dot. Consider the
energy level for each angle of rotation, then drag the dot to the
appropriate energy level and let go to drop.
All points must be moved. Click and drag each point to the
appropriate energy level for each Newman projection.
Potential Energy
100
90
80
70
60
50
40
30
20
10
0
60
120
180
240
Angle of internal rotation (dihedral angle)
300
360
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
