A solubility curve for KCI in water as a function of temperature is below. Potassium chloride and sodium chloride are similar in charge and they both have the same anion. Sodium chloride has a solubility of 4.1 g/10 mL H20 at 100 °C. Which salt, potassium chloride or sodium chloride, would have higher solubility at 100 °C? Temperature Dependence of KCI Solubility 6.0 5.5 E 50 4.5 3.5 3.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 B0.0 90.0 100.0 Temperature, C Select one: O Both salts have the same solubility at 100°C O sodium chloride O A comparison can not be determined from the information given. O potassium chloride Mass ka (grams) per 10 ml. H,0
A solubility curve for KCI in water as a function of temperature is below. Potassium chloride and sodium chloride are similar in charge and they both have the same anion. Sodium chloride has a solubility of 4.1 g/10 mL H20 at 100 °C. Which salt, potassium chloride or sodium chloride, would have higher solubility at 100 °C? Temperature Dependence of KCI Solubility 6.0 5.5 E 50 4.5 3.5 3.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 B0.0 90.0 100.0 Temperature, C Select one: O Both salts have the same solubility at 100°C O sodium chloride O A comparison can not be determined from the information given. O potassium chloride Mass ka (grams) per 10 ml. H,0
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 91AE: The solubility of benzoic acid (HC7H5O2), is 0.34 g/100 mL in water at 25C and is 10.0 g/100 mL in...
Related questions
Question
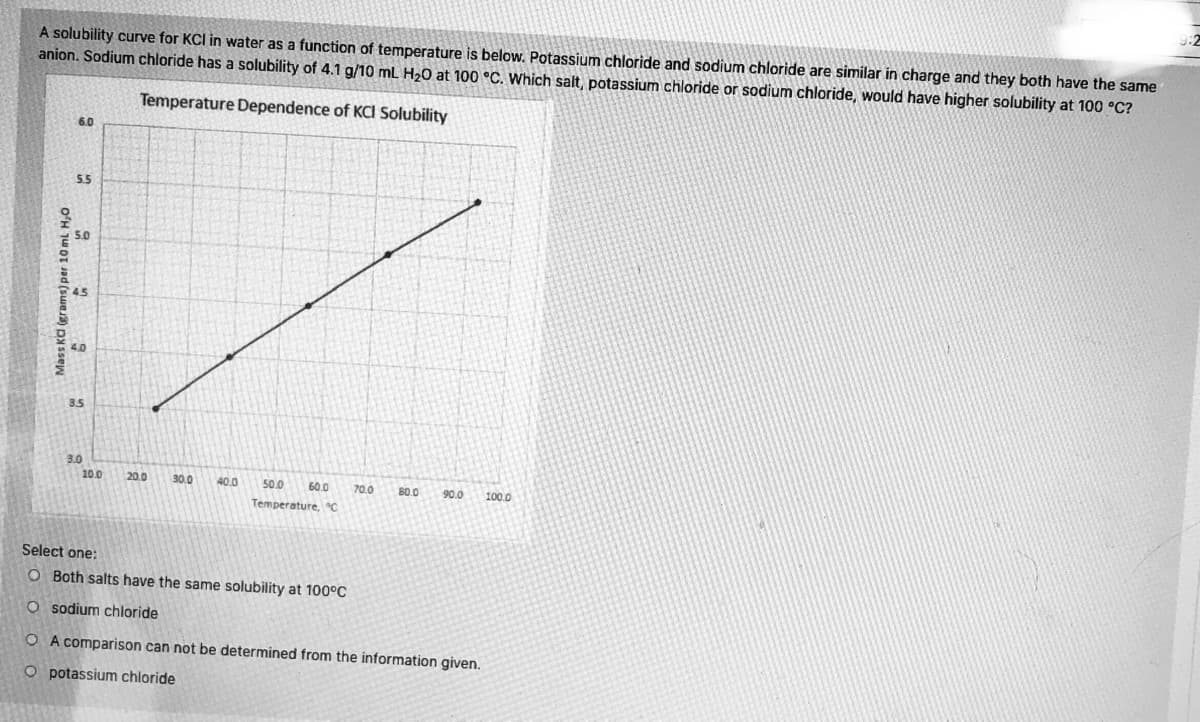
Transcribed Image Text:A solubility curve for KCI in water as a function of temperature is below. Potassium chloride and sodium chloride are similar in charge and they both have the same
anion. Sodium chloride has a solubility of 4.1 g/10 mL H20 at 100 °C. Which salt, potassium chloride or sodium chloride, would have higher solubility at 100 °C?
Temperature Dependence of Ka Solubility
6.0
5.5
5.0
4.5
4.0
3.5
3.0
10.0
20.0
30.0
40.0
50.0
60.0
70.0
80.0
90.0
100.0
Temperature, °C
Select one:
O Both salts have the same solubility at 100°C
O sodium chloride
O A comparison can not be determined from the information given.
O potassium chloride
Mass kd (grams) per 10 ml H,0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT