A solution may contain, chloride, carbonate, phosphate, and or sulfate ions. No effect is observed upon addition of 6m HNO3; this resulting solution will be referred to as solution 1. No effect is observed on addition of 0.1 AGNO3 to solution 1. A white precipitate is reported on addition of 1M BaCl2 to solution 1. Finally, a yellow precipitate is observed on addition of 0.5 M (NH4)8M07O24 to solution. Which of the ions are present, which are absent, and which remain undetermined? State your reasoning.
A solution may contain, chloride, carbonate, phosphate, and or sulfate ions. No effect is observed upon addition of 6m HNO3; this resulting solution will be referred to as solution 1. No effect is observed on addition of 0.1 AGNO3 to solution 1. A white precipitate is reported on addition of 1M BaCl2 to solution 1. Finally, a yellow precipitate is observed on addition of 0.5 M (NH4)8M07O24 to solution. Which of the ions are present, which are absent, and which remain undetermined? State your reasoning.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter35: Spot Tests For Some Common Anions
Section: Chapter Questions
Problem 2ASA
Related questions
Question
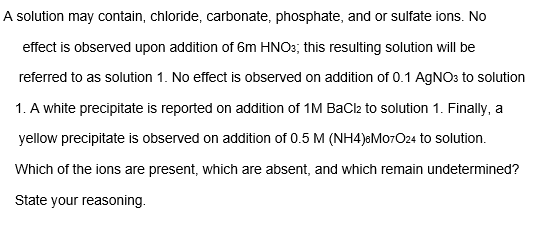
Transcribed Image Text:A solution may contain, chloride, carbonate, phosphate, and or sulfate ions. No
effect is observed upon addition of 6m HNO3; this resulting solution will be
referred to as solution 1. No effect is observed on addition of 0.1 AGNO: to solution
1. A white precipitate is reported on addition of 1M BaCl2 to solution 1. Finally, a
yellow precipitate is observed on addition of 0.5 M (NH4)&M07O24 to solution.
Which of the ions are present, which are absent, and which remain undetermined?
State your reasoning.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

