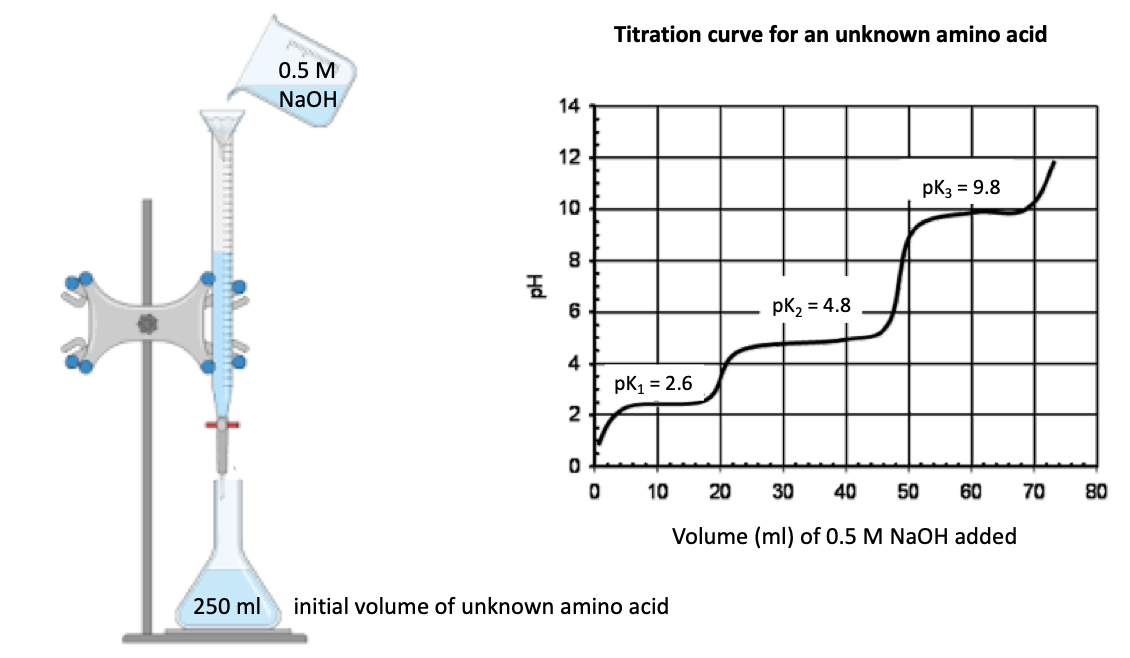
A student added a total of 75 mL sodium hydroxide to titrate an amino acid solution of unknown concentration. They also calculated that they added a total of 14 mL of sodium hydroxide to arrive at pH 2.6 and a total of 32 mL of sodium hydroxide to arrvive at pH 4.8. Information about the molarity of the sodium hydroxide solution used and the initial volume of the unknown amino acid can be derived from the image. What was the initial concentration of the amino acid solution in milimolar (mM)? The calculations must be presented such that those can be recapitulated by the marker. Provide the answer with no decimal places. Show all details of your working out. State the answer in a full sentence
A student added a total of 75 mL sodium hydroxide to titrate an amino acid solution of unknown concentration. They also calculated that they added a total of 14 mL of sodium hydroxide to arrive at pH 2.6 and a total of 32 mL of sodium hydroxide to arrvive at pH 4.8. Information about the molarity of the sodium hydroxide solution used and the initial volume of the unknown amino acid can be derived from the image.
What was the initial concentration of the amino acid solution in milimolar (mM)? The calculations must be presented such that those can be recapitulated by the marker. Provide the answer with no decimal places. Show all details of your working out. State the answer in a full sentence
Trending now
This is a popular solution!
Step by step
Solved in 3 steps









