A student dissolves 13.7 g of sodium chloride (NaCl) in 300. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 21.0 °C to 20.4 °C over the course of 6.9 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaCl(s) → Na' (aq) + Cl (aq) olo You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 1 significant digit. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. I kJ kJ Calculate the reaction enthalpy AHn per mole of NaCl. гxn mol
A student dissolves 13.7 g of sodium chloride (NaCl) in 300. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 21.0 °C to 20.4 °C over the course of 6.9 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaCl(s) → Na' (aq) + Cl (aq) olo You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 1 significant digit. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. I kJ kJ Calculate the reaction enthalpy AHn per mole of NaCl. гxn mol
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 84AP
Related questions
Question
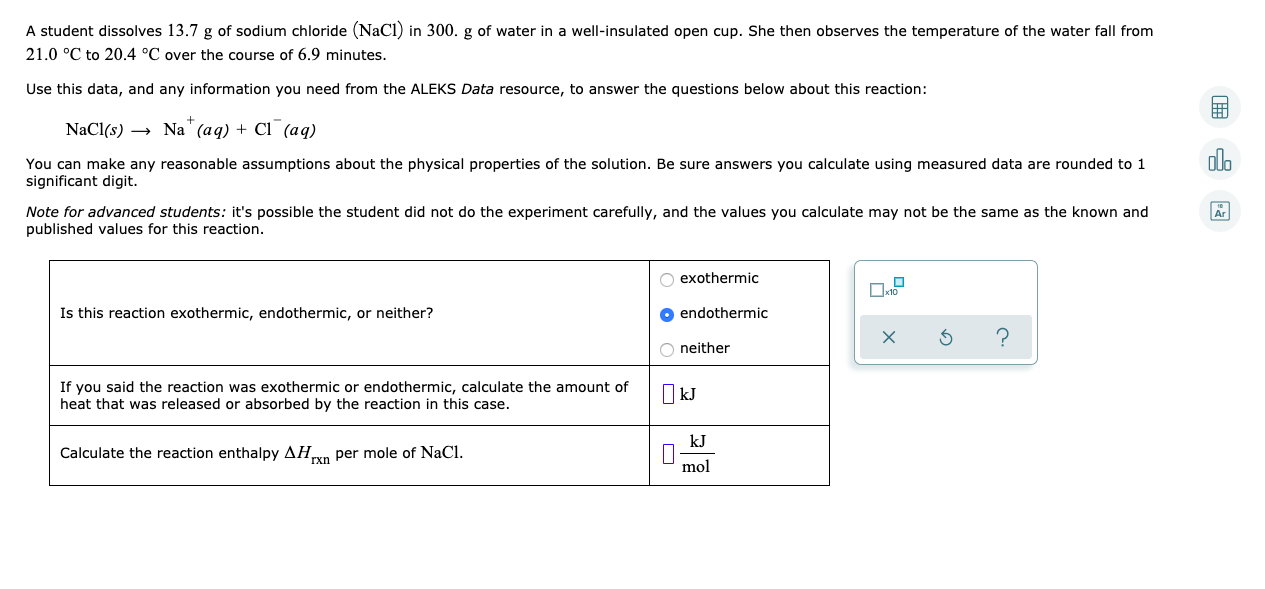
Transcribed Image Text:A student dissolves 13.7 g of sodium chloride (NaCl) in 300. g of water in a well-insulated open cup. She then observes the temperature of the water fall from
21.0 °C to 20.4 °C over the course of 6.9 minutes.
Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction:
NaCl(s) → Na' (aq) + Cl (aq)
olo
You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 1
significant digit.
Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and
published values for this reaction.
O exothermic
Is this reaction exothermic, endothermic, or neither?
O endothermic
O neither
If you said the reaction was exothermic or endothermic, calculate the amount of
heat that was released or absorbed by the reaction in this case.
I kJ
kJ
Calculate the reaction enthalpy AHn per mole of NaCl.
гxn
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER