
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 13QAP
The combustion of methane, 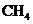 is an exothermic process. Therefore, the products of this reaction must possess (higher/ lower) total potential energy than do the reactants.
is an exothermic process. Therefore, the products of this reaction must possess (higher/ lower) total potential energy than do the reactants.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 10 Solutions
Introductory Chemistry: A Foundation
Ch. 10.1 - at if energy were not conserved? How would this...Ch. 10.4 - u are calculating in a chemistry problem. What if...Ch. 10.5 - ercise 10.1 How many calories of energy correspond...Ch. 10.5 - ercise 10.2 Calculate the joules of energy...Ch. 10.5 - ercise 10.3 A 5.63-g sample of solid gold is...Ch. 10.5 - ercise 10.4 A 2.8-g sample of pure metal requires...Ch. 10.6 - Prob. 10.5SCCh. 10.7 - at if Hess’s law were not true? What are some...Ch. 10.7 - Prob. 10.6SCCh. 10.9 - Prob. 1CT
Ch. 10.10 - at if the first law of thermodynamics was true,...Ch. 10 - Prob. 1ALQCh. 10 - friend of yours reads that the process of water...Ch. 10 - ou place hot metal into a beaker of cold water. ol...Ch. 10 - Prob. 4ALQCh. 10 - Prob. 5ALQCh. 10 - xplain why aluminum cans make good storage...Ch. 10 - n Section 10.7, two characteristics of enthalpy...Ch. 10 - Prob. 8ALQCh. 10 - hat is meant by the term driving forces? Why are...Ch. 10 - Prob. 10ALQCh. 10 - Explain in your own words what is meant by the...Ch. 10 - Prob. 12ALQCh. 10 - What if energy was not conserved? How would this...Ch. 10 - The internal energy of a system is said to be the...Ch. 10 - Hydrogen gas and oxygen gas react violently to...Ch. 10 - Consider four 100.0-g samples of water, each in a...Ch. 10 - For each of the following situations ac. use the...Ch. 10 - Prob. 18ALQCh. 10 - Does the entropy of the system increase or...Ch. 10 - Prob. 20ALQCh. 10 - Prob. 1QAPCh. 10 - Prob. 2QAPCh. 10 - Prob. 3QAPCh. 10 - Prob. 4QAPCh. 10 - Prob. 5QAPCh. 10 - n Fig. 10.1, what kind of energy does ball A...Ch. 10 - Prob. 7QAPCh. 10 - f you spilled a cup of freshly brewed hot tea on...Ch. 10 - Prob. 9QAPCh. 10 - Prob. 10QAPCh. 10 - In studying heat flows for chemical processes,...Ch. 10 - When a chemical system evolves energy, where does...Ch. 10 - The combustion of methane, is an exothermic...Ch. 10 - Are the following processes exothermic or...Ch. 10 - What do we mean by thermodynamics? What is the...Ch. 10 - Prob. 16QAPCh. 10 - Prob. 17QAPCh. 10 - If q for a process is a positive number, then the...Ch. 10 - For an endothermic process, q will have a...Ch. 10 - A system absorbs 215 kJ of heat, and 116 kJ of...Ch. 10 - Prob. 21QAPCh. 10 - Prob. 22QAPCh. 10 - If 8.40 kJ of heat is needed to raise the...Ch. 10 - If it takes 654 J of energy to warm a 5.51-g...Ch. 10 - Prob. 25QAPCh. 10 - Prob. 26QAPCh. 10 - Covert the following numbers of kilojoules into...Ch. 10 - Prob. 28QAPCh. 10 - Prob. 29QAPCh. 10 - Prob. 30QAPCh. 10 - .5 kJ of heat is applied to a 1012-g block of...Ch. 10 - What quantity of heat energy must have en applied...Ch. 10 - If 125 J of heat energy is applied to a block of...Ch. 10 - If 100. J of heat energy is applied to a 25-g...Ch. 10 - What quantity of heat is required to raise the...Ch. 10 - Prob. 36QAPCh. 10 - The “Chemistry in Focus” segment Nature Has Hot...Ch. 10 - In the “Chemistry in Focus” segment Firewalking:...Ch. 10 - Prob. 39QAPCh. 10 - A _________ is a device used to determine the heat...Ch. 10 - The enthalpy change for the reaction of hydrogen...Ch. 10 - For the reaction kJ per mole of formed. Calculate...Ch. 10 - Prob. 43QAPCh. 10 - When ethanol (grain alcohol, is burned in oxygen,...Ch. 10 - Prob. 45QAPCh. 10 - Prob. 46QAPCh. 10 - Prob. 47QAPCh. 10 - Prob. 48QAPCh. 10 - Prob. 49QAPCh. 10 - Prob. 50QAPCh. 10 - Prob. 51QAPCh. 10 - Prob. 52QAPCh. 10 - Prob. 53QAPCh. 10 - Prob. 54QAPCh. 10 - Prob. 55QAPCh. 10 - Prob. 56QAPCh. 10 - Prob. 57QAPCh. 10 - Prob. 58QAPCh. 10 - Prob. 59QAPCh. 10 - Prob. 60QAPCh. 10 - If a reaction occurs readily but has an...Ch. 10 - Prob. 62QAPCh. 10 - Prob. 63QAPCh. 10 - Prob. 64QAPCh. 10 - Which of the following is an endothermic process?...Ch. 10 - Prob. 66APCh. 10 - Prob. 67APCh. 10 - Calculate the amount of energy required (in...Ch. 10 - If takes 1.25 kJ of energy to heat a certain...Ch. 10 - What quantity of heat energy would have to be...Ch. 10 - The specific heat capacity of gold is 0.13 J/g °C....Ch. 10 - Calculate the amount of energy required (in...Ch. 10 - If 10. J of heat is applied to 5.0-g samples of...Ch. 10 - A 50.1)-g sample of water at 100. °C is poured...Ch. 10 - A 25.0-g sample of pure iron at 85 °C is dropped...Ch. 10 - If 7.24 kJ of heat is applied to a 952-g block of...Ch. 10 - For each of the substances listed in Table 10.1,...Ch. 10 - A system releases 213 kJ of heat and has a...Ch. 10 - Prob. 79APCh. 10 - Calculate the enthalpy change when 5.00 g of...Ch. 10 - Prob. 81APCh. 10 - Prob. 82APCh. 10 - It has been determined that the body can generate...Ch. 10 - Prob. 84APCh. 10 - Prob. 85CPCh. 10 - The specific heat capacity of graphite is 0.71 J/g...Ch. 10 - A swimming pool, 10.0 in by 4.0 m, is filled with...Ch. 10 - Prob. 88CPCh. 10 - Prob. 89CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Define heat. What are its units? How does it differ from energy?arrow_forwardDescribe the interconversions of potential and kinetic energy in a moving pendulum. A moving pendulum eventually comes to rest. Has the energy been lost? If not, what has happened to it?arrow_forwardIn the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)H=24.8kJ The enthalpy change for the combustion of carbon monoxide is 2CO(g)+O2(g)2CO2(g)H=566kJ Use this information to calculate the enthalpy change for the equation 4Fe(s)+3O2(g)2Fe2O3(s)H=?arrow_forward
- Determine whether the statements given below are true or false. Consider an endothermic process taking place in a beaker at room temperature. (a) Heat flows from the surroundings to the system. (b) The beaker is cold to the touch. (c) The pressure of the system decreases. (d) The value of q for the system is positive.arrow_forwardWhen 2.50 g of methane burns in oxygen, 125 kJ of heat is produced. What is the enthalpy of combustion per mole of methane under these conditions?arrow_forwardWhat mass of acetylene, C2H2(g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is 1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine which substance produces more heat per gram.arrow_forward
- A small car is traveling at twice the speed of a larger car, which has twice the mass of the smaller car. Which car has the greater kinetic energy? (Or do they both have the same kinetic energy?)arrow_forwardDetermine whether the statements given below are true or false. Consider enthalpy (H). (a) It is a state property. (b) qreaction(atconstantP)=H=HproductsHreactants (c) The magnitude of H is independent of the amount of reactant. (d) In an exothermic process, the enthalpy of the system remains unchanged.arrow_forwardConsider the following reaction in the vessel described in Question 57. A(g)+B(g)C(s)For this reaction, E=286 J, the piston moves up and the system absorbs 388 J of heat from its surroundings. (a) Is work done by the system? (b) How much work?arrow_forward
- Consider the combustion of propane: C3H8(g)+5O2(g)3CO2(g)+4H2O(l)H=2221KJ Assume that all the heat in Example 7-3 comes from the combustion of propane. What mass of propane must be burned to furnish this amount of energy assuming the heat transfer process is 60.% efficient?arrow_forwardAn exothermic reaction is carried out in a coffee-cup calorimeter. Which of the following statements is/are NOT true for the process? (a) The temperature of the water increases. (b) Heat is absorbed by the water. (c) The enthalpy of the products is higher than the enthalpy of the reactants. (d) qH2o=qrxn (e) qrxn0 (f) qrxn+qH2o=0arrow_forwardNatural gas companies in the United States use the therm as a unit of energy. One therm is 1105 BTU. (a) How many joules are in one therm? (1J=9.48104BTU) (b) When propane gas, C3H8, is burned in oxygen, CO2 and steam are produced. How many therms of energy are given off by 1.00 mol of propane gas?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY