A student needs to prepare 250 mL of a 0.500 M aqueous solution of sucrose, C,,H,,0, (aq), which is used frequently in biological experiments. 200 mL 250 mL 150 200 100 150 50 100 250 mL B Which type of glassware should be used to make this How should the correct amount of solute be obtained? solution (assuming that the accuracy of the concentration is important)? O Measure out x mo! of solid sucrose on a molemeter. O Measure out x g of solid sucrose on a balance. Measure out x cm of sucrose with a ruler. OC Based on the selected correct unit, what is the value of x? X3D
A student needs to prepare 250 mL of a 0.500 M aqueous solution of sucrose, C,,H,,0, (aq), which is used frequently in biological experiments. 200 mL 250 mL 150 200 100 150 50 100 250 mL B Which type of glassware should be used to make this How should the correct amount of solute be obtained? solution (assuming that the accuracy of the concentration is important)? O Measure out x mo! of solid sucrose on a molemeter. O Measure out x g of solid sucrose on a balance. Measure out x cm of sucrose with a ruler. OC Based on the selected correct unit, what is the value of x? X3D
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 101AE: You wish to prepare 1 L of a 0.02-M potassium iodate solution. You require that the final...
Related questions
Question
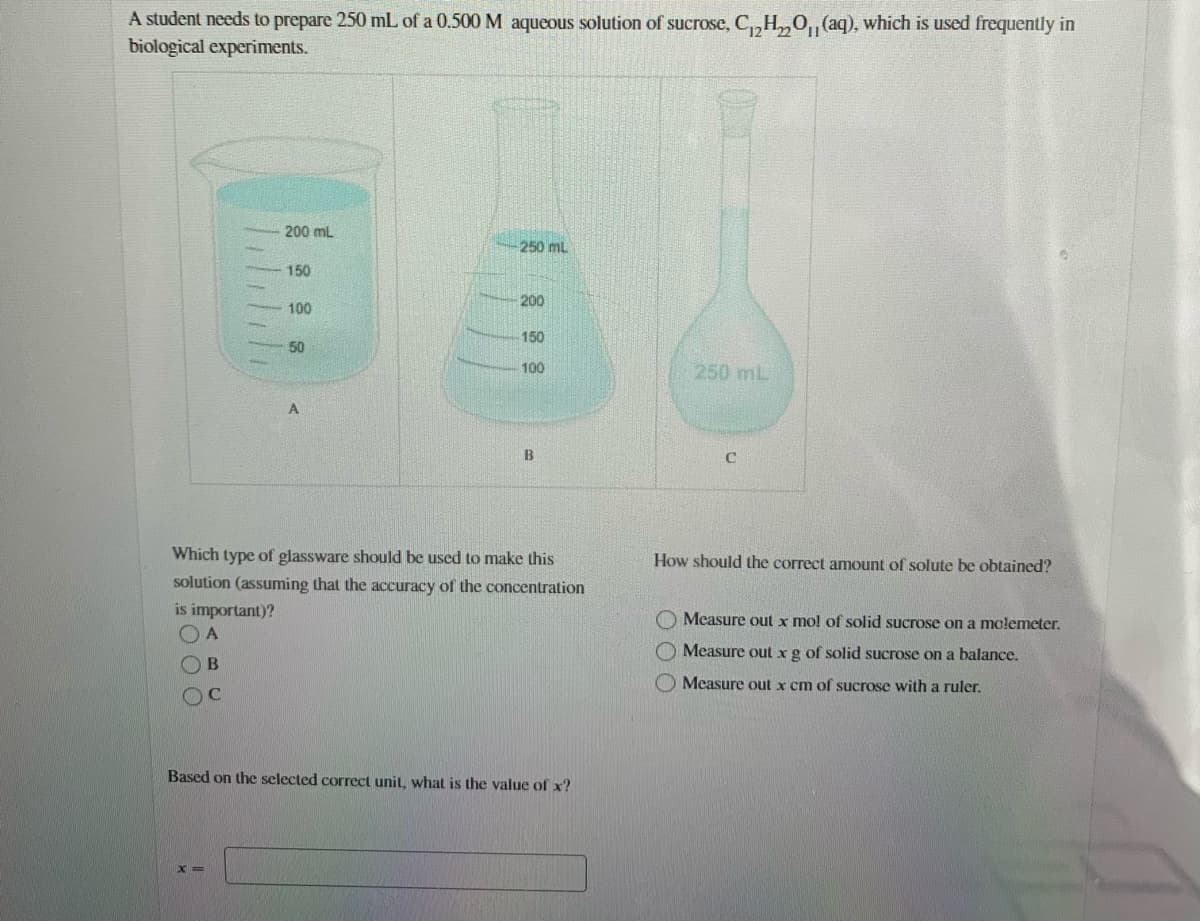
Transcribed Image Text:A student needs to prepare 250 mL of a 0.500 M aqueous solution of sucrose, C,,H,0, (aq), which is used frequently in
biological experiments.
200 mL
250 ml
150
200
100
150
50
100
250 mL
Which type of glassware should be used to make this
How should the correct amount of solute be obtained?
solution (assuming that the accuracy of the concentration
is important)?
O Measure out x mo! of solid sucrose on a molemeter.
O Measure out xg of solid sucrose on a balance.
OB
O Measure out x cm of sucrose with a ruler.
Based on the selected correct unit, what is the value of x?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning