A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 747 mm Hg. Express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. Hint: 1 atm 101.3 kPa = 760 tor = 760 mm Hg = 14.69 psi = 1.013x105 Pa mm Hg atm kPa torr psi Pa 747 Submit Answer Retry Entire Group 9 more group attempts remaining
A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 747 mm Hg. Express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. Hint: 1 atm 101.3 kPa = 760 tor = 760 mm Hg = 14.69 psi = 1.013x105 Pa mm Hg atm kPa torr psi Pa 747 Submit Answer Retry Entire Group 9 more group attempts remaining
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.22E: Earths atmosphere is approximately 80 N2 and 20 O2. If the total atmospheric pressure at sea level...
Related questions
Question
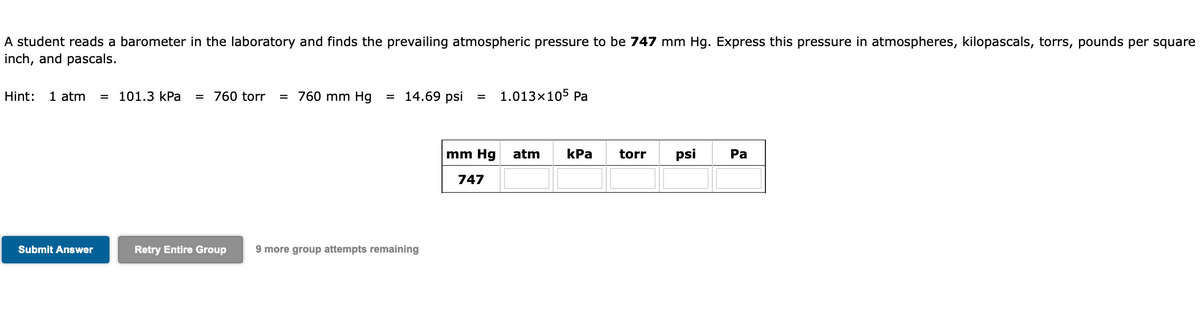
Transcribed Image Text:A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 747 mm Hg. Express this pressure in atmospheres, kilopascals, torrs, pounds per square
inch, and pascals.
Hint:
1 atm
= 101.3 kPa
= 760 torr
= 760 mm Hg
= 14.69 psi
1.013×105 Pa
mm Hg
atm
kPa
torr
psi
Ра
747
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning