A student runs two experiments with a constant-volume "bomb" calorimeter containing 1000. g of water (see sketch at thermometer right). stirrer First, a 7.000 g tablet of benzoic acid (C,H,CO,H) is put into the "bomb" and burned completely in an excess of water oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is insulation observed to rise from 23.00 °C to 62.40 °C over a time of 5.5 minutes. Next, 4.300 g of ethylene (C,H,) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 23.00 °C to 63.99 °C. chemical reaction "bomb" Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: A "bomb" calorimeter. C,H,(g) + 30,(g) 2Co,(g) + 2 H,0 (g) Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not exactly match published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in the second experiment. kJ kJ Calculate the reaction enthalpy AH per mole of C,H4. rxn mol
A student runs two experiments with a constant-volume "bomb" calorimeter containing 1000. g of water (see sketch at thermometer right). stirrer First, a 7.000 g tablet of benzoic acid (C,H,CO,H) is put into the "bomb" and burned completely in an excess of water oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is insulation observed to rise from 23.00 °C to 62.40 °C over a time of 5.5 minutes. Next, 4.300 g of ethylene (C,H,) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 23.00 °C to 63.99 °C. chemical reaction "bomb" Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: A "bomb" calorimeter. C,H,(g) + 30,(g) 2Co,(g) + 2 H,0 (g) Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not exactly match published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in the second experiment. kJ kJ Calculate the reaction enthalpy AH per mole of C,H4. rxn mol
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.132QP
Related questions
Question
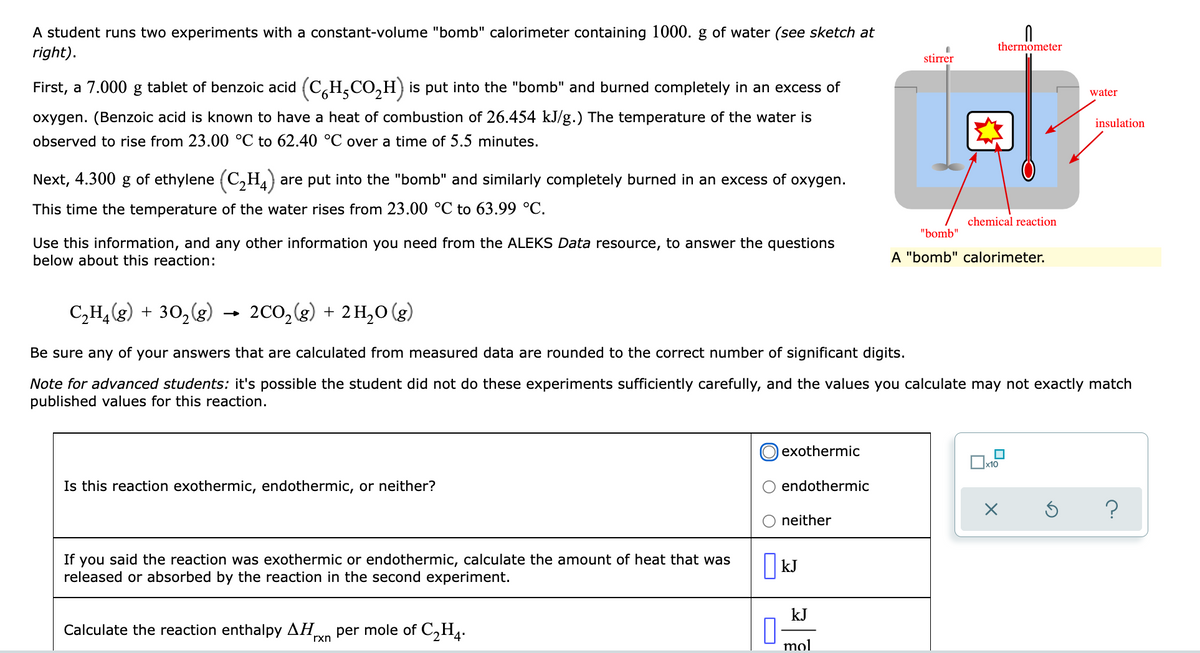
Transcribed Image Text:A student runs two experiments with a constant-volume "bomb" calorimeter containing 1000. g of water (see sketch at
thermometer
right).
stirrer
First, a 7.000 g tablet of benzoic acid (C,H,CO,H) is put into the "bomb" and burned completely in an excess of
water
oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is
insulation
observed to rise from 23.00 °C to 62.40 °C over a time of 5.5 minutes.
Next, 4.300 g of ethylene (C,H4)
are put into the "bomb" and similarly completely burned in an excess of oxygen.
This time the temperature of the water rises from 23.00 °C to 63.99 °C.
chemical reaction
"bomb"
Use this information, and any other information you need from the ALEKS Data resource, to answer the questions
below about this reaction:
A "bomb" calorimeter.
C,H,(g) + 30,(g)
2C0,(g) + 2 H,0 (g)
Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits.
Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not exactly match
published values for this reaction.
exothermic
x10
Is this reaction exothermic, endothermic, or neither?
endothermic
neither
If you said the reaction was exothermic or endothermic, calculate the amount of heat that was
released or absorbed by the reaction in the second experiment.
||kJ
kJ
Calculate the reaction enthalpy AH_
per mole of C,H4.
rxn
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning