A student sets up a voltaic cell using a 1 M zinc sulfate solution with a zinc electrode and a 1 M tin(1I) nitrate solution with a tin electrode, connecting the two cells with a salt bridge. The student connects the red lead to the zinc electrode and the black lead to the tin electrode. The voltmeter reads a voltage of -0.62 V. -0.62 Zn Sn 1M 1M Zn2+ Sn2+ Based on the student's experimental setup and voltage measurement, which of these statements is true? Select the single best answer. A. The identity of the metal electrodes should not match the aqueous metal ion in each solution. B. The leads are not connected correctly. C. The salt bridge causes the voltage to be negative. D. The voltage difference between zinc and tin should be 0.00 volts.
A student sets up a voltaic cell using a 1 M zinc sulfate solution with a zinc electrode and a 1 M tin(1I) nitrate solution with a tin electrode, connecting the two cells with a salt bridge. The student connects the red lead to the zinc electrode and the black lead to the tin electrode. The voltmeter reads a voltage of -0.62 V. -0.62 Zn Sn 1M 1M Zn2+ Sn2+ Based on the student's experimental setup and voltage measurement, which of these statements is true? Select the single best answer. A. The identity of the metal electrodes should not match the aqueous metal ion in each solution. B. The leads are not connected correctly. C. The salt bridge causes the voltage to be negative. D. The voltage difference between zinc and tin should be 0.00 volts.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 77AP
Related questions
Question
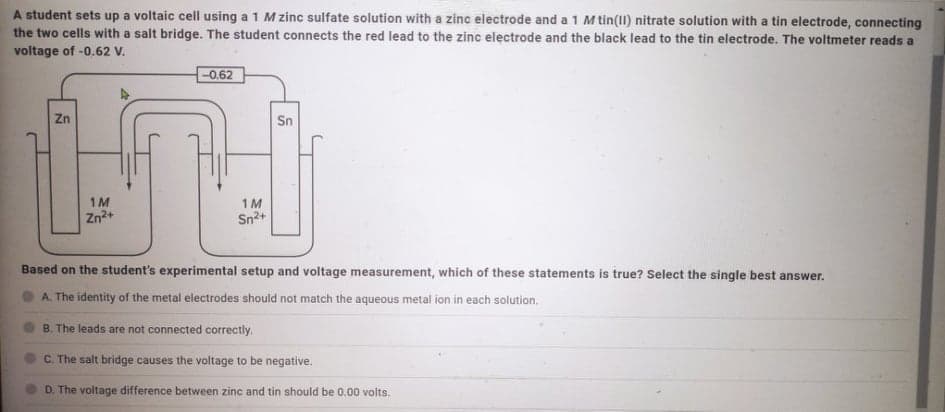
Transcribed Image Text:A student sets up a voltaic cell using a 1 M zinc sulfate solution with a zinc electrode and a 1 M tin(II) nitrate solution with a tin electrode, connecting
the two cells with a salt bridge. The student connects the red lead to the zinc electrode and the black lead to the tin electrode. The voltmeter reads a
voltage of -0.62 V.
0.62
Zn
Sn
1M
1M
Zn2+
Sn2+
Based on the student's experimental setup and voltage measurement, which of these statements is true? Select the single best answer.
A. The identity of the metal electrodes should not match the aqueous metal ion in each solution.
B. The leads are not connected correctly.
C. The salt bridge causes the voltage to be negative.
D. The voltage difference between zinc and tin should be 0.00 volts.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
