A tank contains 1000 L of pure water. Brine that contains 0.05 kg of salt per liter of water enters the tank at a rate of 5 L/min. Separately, brine that contains 0.04 kg of salt per liter of water enters the tank at a rate of 10 L/min. The solution is kept thoroughly mixed and drains from the tank at a rate of 15 L/min. Let y(t) be the amount of salt (in kg) aftert minutes. (a) How much salt is in the tank after t minutes? y(t) kg (b) How much salt is in the tank after 30 minutes? (Round the answer to one decimal place.) y(30) = kg
A tank contains 1000 L of pure water. Brine that contains 0.05 kg of salt per liter of water enters the tank at a rate of 5 L/min. Separately, brine that contains 0.04 kg of salt per liter of water enters the tank at a rate of 10 L/min. The solution is kept thoroughly mixed and drains from the tank at a rate of 15 L/min. Let y(t) be the amount of salt (in kg) aftert minutes. (a) How much salt is in the tank after t minutes? y(t) kg (b) How much salt is in the tank after 30 minutes? (Round the answer to one decimal place.) y(30) = kg
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.86E
Related questions
Question
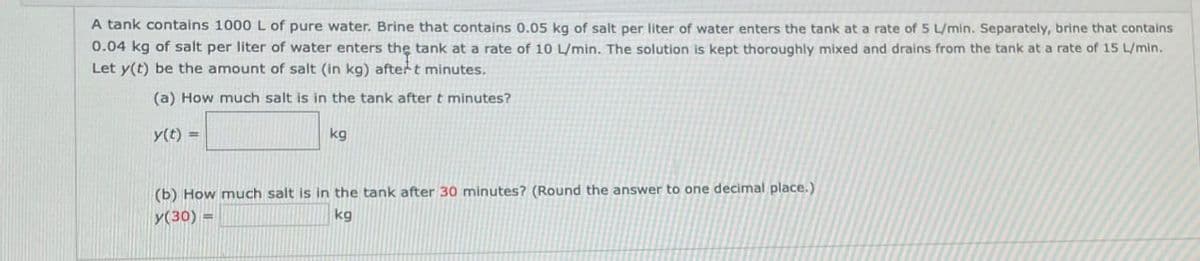
Transcribed Image Text:A tank contains 1000 L of pure water. Brine that contains 0.05 kg of salt per liter of water enters the tank at a rate of 5 L/min. Separately, brine that contains
0.04 kg of salt per liter of water enters the tank at a rate of 10 L/min. The solution is kept thoroughly mixed and drains from the tank at a rate of 15 L/min.
Let y(t) be the amount of salt (in kg) aftert minutes.
(a) How much salt is in the tank after t minutes?
y(t)
kg
(b) How much salt is in the tank after 30 minutes? (Round the answer to one decimal place.)
y(30) =
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning