(a) Using Hückel's method, describe the energy of the molecular orbitals T of cyclobutadiene C4H4, which is a flat molecule made up of a square ring of carbons, as shown in the figure; (b) Do the same for 1,3-butadiene C4H6, which is an elongated planar molecule, as shown in the figure. (c) In both cases, draw an energy diagram indicating the filling of the orbitals and identifying the HOMO and the LUMO. Sketch by hand schematically (using two colors to distinguish the lobes from the 2pz orbitals) the result obtained for the orbitals, identifying them with the corresponding energy. If possible, you can use the Orbital Viewer program to plot the orbitals.
(a) Using Hückel's method, describe the energy of the molecular orbitals T of cyclobutadiene C4H4, which is a flat molecule made up of a square ring of carbons, as shown in the figure; (b) Do the same for 1,3-butadiene C4H6, which is an elongated planar molecule, as shown in the figure. (c) In both cases, draw an energy diagram indicating the filling of the orbitals and identifying the HOMO and the LUMO. Sketch by hand schematically (using two colors to distinguish the lobes from the 2pz orbitals) the result obtained for the orbitals, identifying them with the corresponding energy. If possible, you can use the Orbital Viewer program to plot the orbitals.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 26P: Following the pattern of Figure 6.21, work out the correlation diagram for the BeN molecule, showing...
Related questions
Question
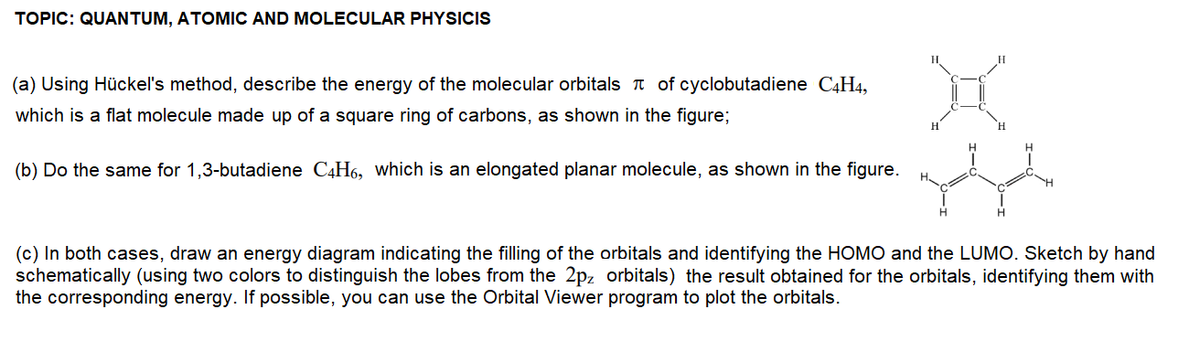
Transcribed Image Text:TOPIC: QUANTUM, ATOMIC AND MOLECULAR PHYSICIS
(a) Using Hückel's method, describe the energy of the molecular orbitals T of cyclobutadiene C4H4,
which is a flat molecule made up of a square ring of carbons, as shown in the figure;
(b) Do the same for 1,3-butadiene C4H6, which is an elongated planar molecule, as shown in the figure.
(c) In both cases, draw an energy diagram indicating the filling of the orbitals and identifying the HOMO and the LUMO. Sketch by hand
schematically (using two colors to distinguish the lobes from the 2pz orbitals) the result obtained for the orbitals, identifying them with
the corresponding energy. If possible, you can use the Orbital Viewer program to plot the orbitals.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning