A voltaic cell is constructed in which the anode is a Fe Fe2* half cell and the cathode is a Co Co2* half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: > The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate v the Co|Co2* electrode v the Fe Fe2+ electrode. In the salt bridge, anions migrate | |the Fe|Fe2+. compartment | v the Co|Co2+ compartment.
A voltaic cell is constructed in which the anode is a Fe Fe2* half cell and the cathode is a Co Co2* half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: > The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate v the Co|Co2* electrode v the Fe Fe2+ electrode. In the salt bridge, anions migrate | |the Fe|Fe2+. compartment | v the Co|Co2+ compartment.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 105QAP: Consider a voltaic cell in which the following reaction occurs. Zn(s)+Sn2+(aq)Zn2+(aq)+Sn(s) (a)...
Related questions
Question
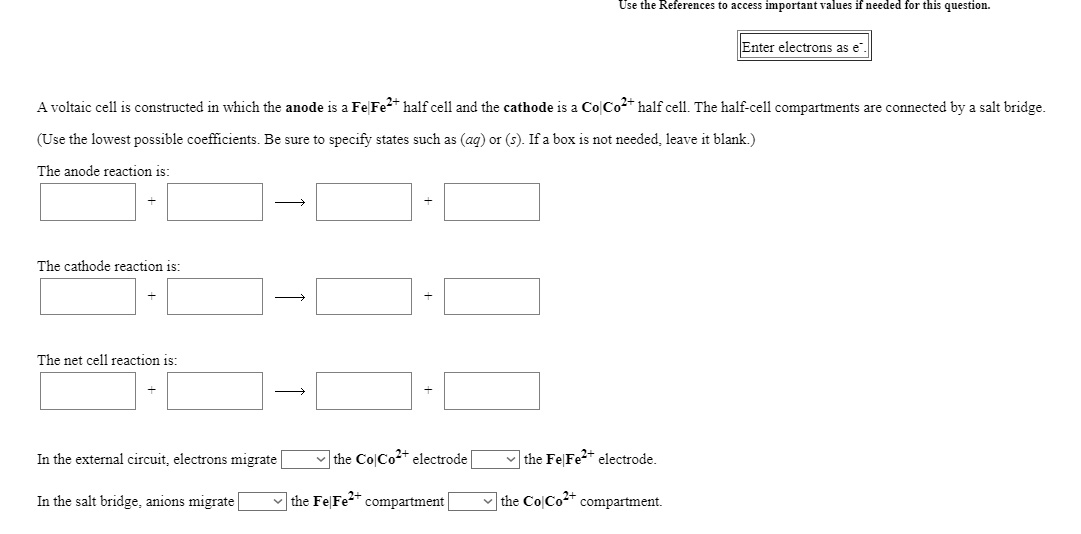
Transcribed Image Text:Use the References to access important values if needed for this question.
Enter electrons as e
A voltaic cell is constructed in which the anode is a FeFe2* half cell and the cathode is a Co|Co2* half cell. The half-cell compartments are connected by a salt bridge.
(Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.)
The anode reaction is:
The cathode reaction is:
The net cell reaction is:
>
In the external circuit, electrons migrate
v the Co|Co2+ electrode
v the Fe|Fe2* electrode.
In the salt bridge, anions migrate
the FelFe2+
compartment
v the Co|Co* compartment.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning