Enter electrons as e". A voltaic cell is constructed in which the anode is a Sn|Sn2* half cell and the cathode is a H2|H* half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate | v the H,|H* clectrode | the Sn|Sn2* electrode. In the salt bridge, anions migrate v the H2|H* compartment| the Sn|Sn+ compartment. + +
Enter electrons as e". A voltaic cell is constructed in which the anode is a Sn|Sn2* half cell and the cathode is a H2|H* half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate | v the H,|H* clectrode | the Sn|Sn2* electrode. In the salt bridge, anions migrate v the H2|H* compartment| the Sn|Sn+ compartment. + +
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell...
Related questions
Question
100%
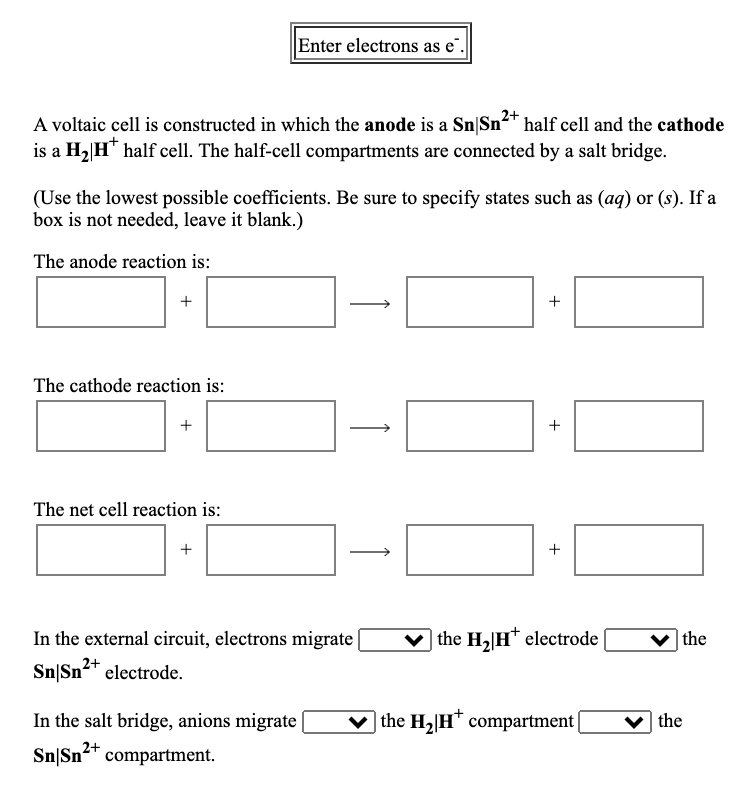
Transcribed Image Text:Enter electrons as e".
2+
A voltaic cell is constructed in which the anode is a Sn|Sn²* half cell and the cathode
is a H2 H" half cell. The half-cell compartments are connected by a salt bridge.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a
box is not needed, leave it blank.)
The anode reaction is:
The cathode reaction is:
The net cell reaction is:
+
In the external circuit, electrons migrate
v
| the H2|H* electrode
|the
Sn|Sn²* electrode.
2+
In the salt bridge, anions migrate
v the H2|H* compartment|
the
Sn|Sn²+ compartment.
+
↑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning