A volume of 40.0 mL of aqueous potassium hydroxide (KOH) was titrated against a standard solution of sulfuric acid (H2SO4). What was the molarity of the KOH solution if 15.2 mL of 1,50 M H2SO4 was needed? The equation is 2KOH(aq) + H2SO4(aq)→K2S04(aq) + 2H2O(1) Express your answer with the appropriate units. , View Available Hint(s) T(mplates Symbols uado redo resat keyboard shortcuts help molarity = Value Units Submit Previous Answers Request Answer x Incorrect; Try Again; 5 attempts remaining - Part B Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. For example, a solution of hydrogen peroxide, H202, can be titrated against a solution of potassium permanganate, KMN04. The following equation represents the reaction: 2KMNO4(aq) + H2O2(aq) + 3H2S04(aq)→ 302(g) + 2MNS04(aq) + K2SO4(aq) + 4H20(1) A certain amount of hydrogen peroxide was dissolved in 100. mL of water and then titrated with 1.68 M KMN04. What mass of H2O2 was dissolved if the titration required 15.3 mL of the KMN04 solution? Express your answer with the appropriate units. , View Available Hint(s) Te(mplates Symbolš uado redo resat keyboard shortcuts help, mass of H2O2 = Value Units Submit
A volume of 40.0 mL of aqueous potassium hydroxide (KOH) was titrated against a standard solution of sulfuric acid (H2SO4). What was the molarity of the KOH solution if 15.2 mL of 1,50 M H2SO4 was needed? The equation is 2KOH(aq) + H2SO4(aq)→K2S04(aq) + 2H2O(1) Express your answer with the appropriate units. , View Available Hint(s) T(mplates Symbols uado redo resat keyboard shortcuts help molarity = Value Units Submit Previous Answers Request Answer x Incorrect; Try Again; 5 attempts remaining - Part B Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. For example, a solution of hydrogen peroxide, H202, can be titrated against a solution of potassium permanganate, KMN04. The following equation represents the reaction: 2KMNO4(aq) + H2O2(aq) + 3H2S04(aq)→ 302(g) + 2MNS04(aq) + K2SO4(aq) + 4H20(1) A certain amount of hydrogen peroxide was dissolved in 100. mL of water and then titrated with 1.68 M KMN04. What mass of H2O2 was dissolved if the titration required 15.3 mL of the KMN04 solution? Express your answer with the appropriate units. , View Available Hint(s) Te(mplates Symbolš uado redo resat keyboard shortcuts help, mass of H2O2 = Value Units Submit
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.107QE
Related questions
Question
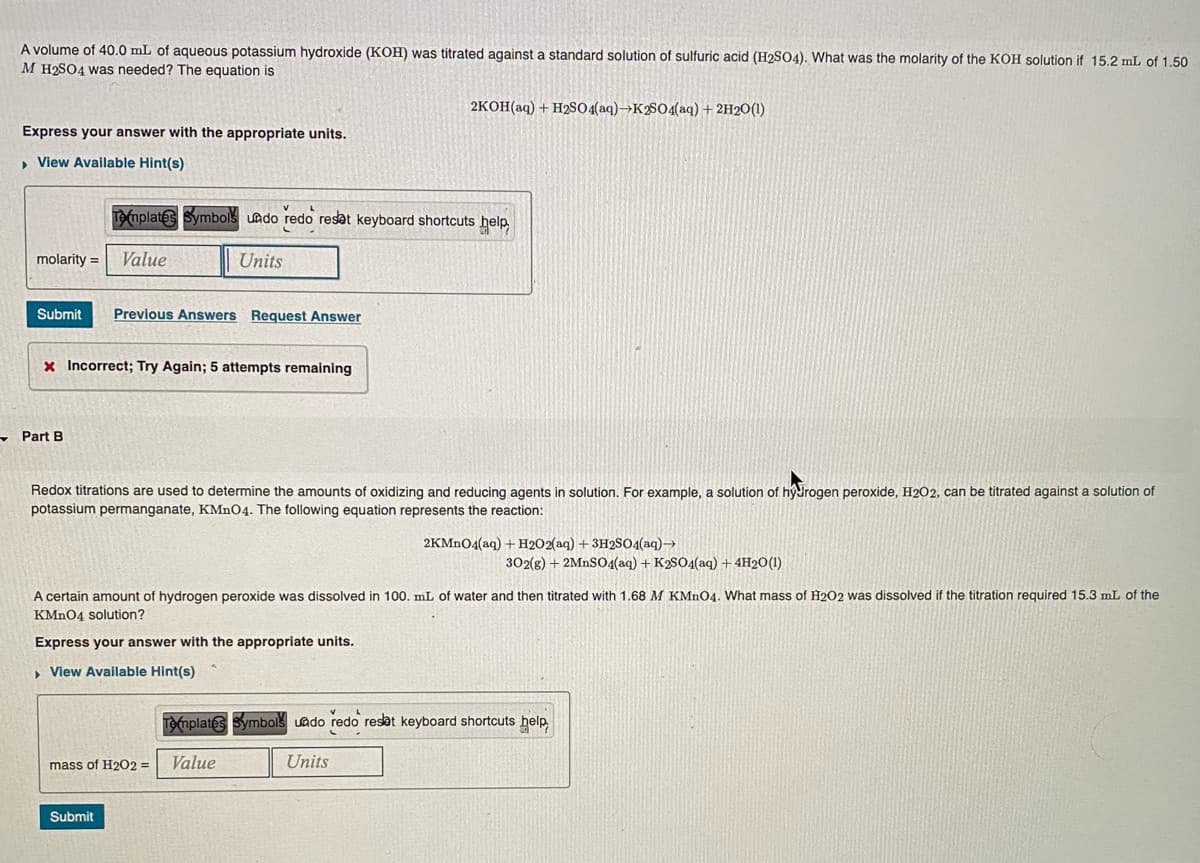
Transcribed Image Text:A volume of 40.0 mL of aqueous potassium hydroxide (KOH) was titrated against a standard solution of sulfuric acid (H2SO4). What was the molarity of the KOH solution if 15.2 mL of 1.50
M H2SO4 was needed? The equation is
2KOH(aq) + H2SO4(aq)→K2804(aq) + 2H2O(1)
Express your answer with the appropriate units.
» View Available Hint(s)
T(mplates Symbols uado redo resat keyboard shortcuts help
molarity =
Value
Units
Submit
Previous Answers Request Answer
x Incorrect; Try Again; 5 attempts remaining
- Part B
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. For example, a solution of hydrogen peroxide, H2O2, can be titrated against a solution of
potassium permanganate, KMN04. The following equation represents the reaction:
2KMN04(aq) + H2O2(aq) + 3H2S04(aq)→
302(g) + 2MNS04(aq) + K2SO4(aq) + 4H20(1)
A certain amount of hydrogen peroxide was dissolved in 100. mL of water and then titrated with 1.68 M KMNO4. What mass of H2O2 was dissolved if the titration required 15.3 mL of the
KMN04 solution?
Express your answer with the appropriate units.
, View Available Hint(s)
Templates Symbolš uado redo resat keyboard shortcuts help,
mass of H2O2 =
Value
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning