A weighed quantity of PCI5(s) is sealed in a 100.0-cm³ glass bulb to which a pressure gauge is attached. The bulb is heated to 250°C, and the gauge shows that the pressure in the bulb rises to 0.895 atm. At this temperature, the solid PCI, is all vaporized and also partially dissociated into Cl2(g) and PCI3(g) according to the equation PCI5(g) 2 PCI3(g) + Cl(g) At 250°C, K = 2.15 for this reaction. Assume that the contents of the bulb are at equilibrium and calculate the partial pressure of the three different chemical species in the vessel.
A weighed quantity of PCI5(s) is sealed in a 100.0-cm³ glass bulb to which a pressure gauge is attached. The bulb is heated to 250°C, and the gauge shows that the pressure in the bulb rises to 0.895 atm. At this temperature, the solid PCI, is all vaporized and also partially dissociated into Cl2(g) and PCI3(g) according to the equation PCI5(g) 2 PCI3(g) + Cl(g) At 250°C, K = 2.15 for this reaction. Assume that the contents of the bulb are at equilibrium and calculate the partial pressure of the three different chemical species in the vessel.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter9: The Gaseous State
Section: Chapter Questions
Problem 33P
Related questions
Question
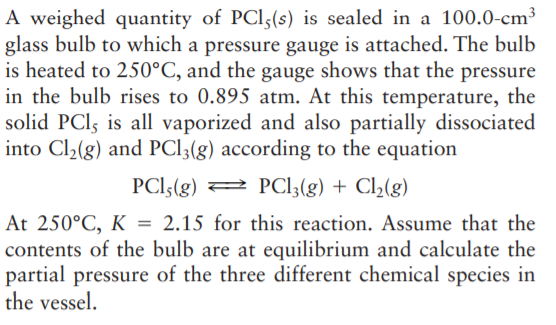
Transcribed Image Text:A weighed quantity of PCI5(s) is sealed in a 100.0-cm³
glass bulb to which a pressure gauge is attached. The bulb
is heated to 250°C, and the gauge shows that the pressure
in the bulb rises to 0.895 atm. At this temperature, the
solid PCI, is all vaporized and also partially dissociated
into Cl2(g) and PCI3(g) according to the equation
PCI5(g) 2 PCI3(g) + Cl(g)
At 250°C, K = 2.15 for this reaction. Assume that the
contents of the bulb are at equilibrium and calculate the
partial pressure of the three different chemical species in
the vessel.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning