(a) What is meant by the term elementary reaction?(b) What is the difference between a unimolecularand a bimolecular elementary reaction? (c) What is a reaction mechanism? (d) What is meant by the term rate-determiningstep?
(a) What is meant by the term elementary reaction?
(b) What is the difference between a unimolecular
and a bimolecular elementary reaction? (c) What is a reaction mechanism? (d) What is meant by the term rate-determining
step?
“Since you have posted a question with multiple sub-parts, we will solve the first three subparts for you. To get the remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.”
Chemical reactions are mainly the process through which reacting molecules (one or more) combines (reacts) and gets transformed into particular substances named as "products". In these reactions, reactants generally rearrange themselves to form products.
(a) Elementary reaction: This reaction mainly occurs in one step. In elementary reaction, chemical species (reactants) get reacted to each other directly and yield product. The reaction possesses only one transition state and product gets formed in single-step always.
(b) Difference between a unimolecular and a bimolecular elementary reaction:
Unimolecular reactions include only single reactant which rearranges itself and yields products. For example: radioactive decay process, carbonation rearrangement, and cis-trans isomerization, and so on. Theses reactions are mainly first-order reactions. The unimolecular reaction example is shown below.
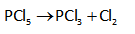
Bimolecular reactions include two reactants that collide with each other and yield products. The two molecules combine or react and exchange groups, atoms or energy. Nucleophilic substitution mainly depicts the bimolecular reaction. The example for a bimolecular reaction is shown below.
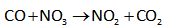
Step by step
Solved in 3 steps with 2 images









