2. The following balanced chemical equation represents a chemical system at equilibrium: Ku C,H,(g) + Br,(g) C,H,Br,(g) colourless brown colourless (a) What visual changes will you see at equilibrium? (b) What is occurring at the molecular level? (c) Is the equilibrium static or dynamic?
2. The following balanced chemical equation represents a chemical system at equilibrium: Ku C,H,(g) + Br,(g) C,H,Br,(g) colourless brown colourless (a) What visual changes will you see at equilibrium? (b) What is occurring at the molecular level? (c) Is the equilibrium static or dynamic?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.84EP: Based on the diagrams, chemical reaction, and reaction conditions depicted in Problem 9-83, which of...
Related questions
Question
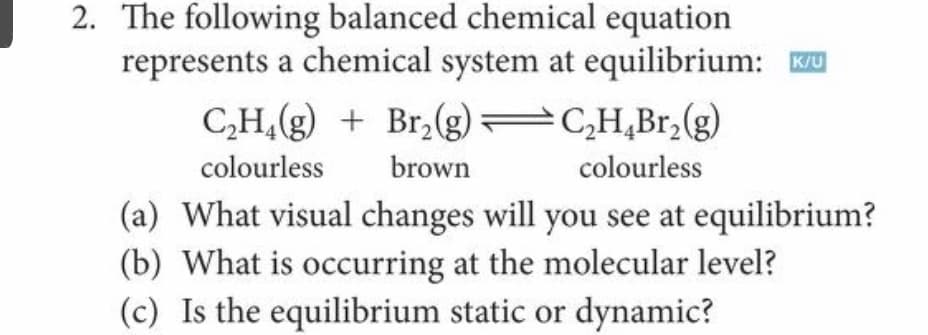
Transcribed Image Text:2. The following balanced chemical equation
represents a chemical system at equilibrium: u
CH,(g) + Br,(g) C,H,Br,(g)
colourless
brown
colourless
(a) What visual changes will you see at equilibrium?
(b) What is occurring at the molecular level?
(c) Is the equilibrium static or dynamic?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning