A. Basicity of Amines, Demonsi Into 3 separate test tubes place approximately 2mL of 1M solutions of ammonium hydroxide, methylamine, and acetamide. Use pH paper to determine the pH of each solution. Record your observation. NH3 Ammonia H3C-NH, Methylamine H;C `NH2 acetamide Question: Write the names of the compound in order of increasing basicity (from least to most basic).
A. Basicity of Amines, Demonsi Into 3 separate test tubes place approximately 2mL of 1M solutions of ammonium hydroxide, methylamine, and acetamide. Use pH paper to determine the pH of each solution. Record your observation. NH3 Ammonia H3C-NH, Methylamine H;C `NH2 acetamide Question: Write the names of the compound in order of increasing basicity (from least to most basic).
Chapter7: Neutralization Titrations And Graphical Representations
Section: Chapter Questions
Problem 15P
Related questions
Question
Answer all questions
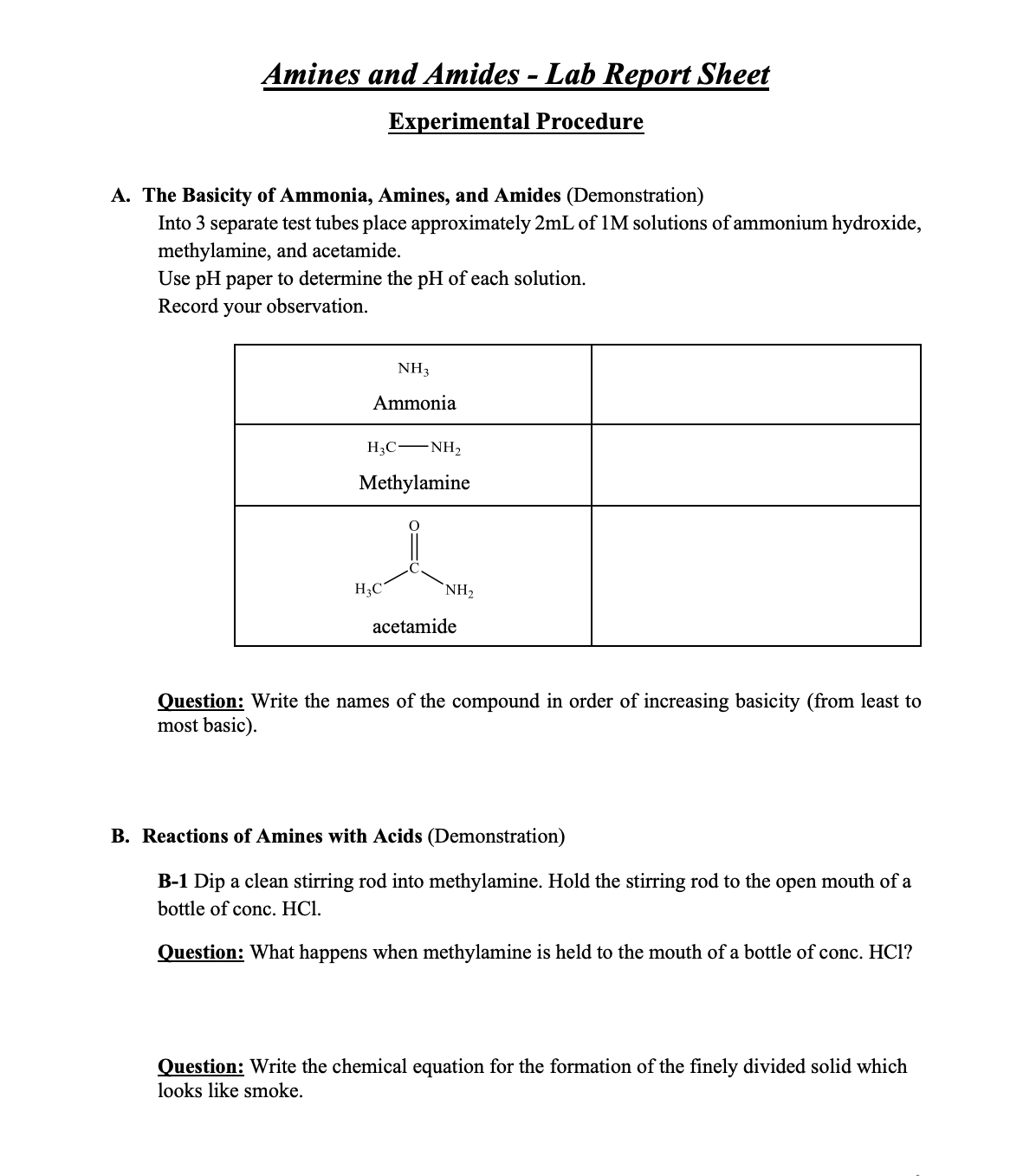
Transcribed Image Text:Amines and Amides - Lab Report Sheet
Experimental Procedure
A. The Basicity of Ammonia, Amines, and Amides (Demonstration)
Into 3 separate test tubes place approximately 2mL of 1M solutions of ammonium hydroxide,
methylamine, and acetamide.
Use pH paper to determine the pH of each solution.
Record your observation.
NH3
Ammonia
H3C -NH2
Methylamine
H3C
NH,
acetamide
Question: Write the names of the compound in order of increasing basicity (from least to
most basic).
B. Reactions of Amines with Acids (Demonstration)
B-1 Dip a clean stirring rod into methylamine. Hold the stirring rod to the open mouth of a
bottle of conc. HCl.
Question: What happens when methylamine is held to the mouth of a bottle of conc. HCl?
Question: Write the chemical equation for the formation of the finely divided solid which
looks like smoke.

Transcribed Image Text:B-2 To approximately 5mL of distilled water in a small beaker add 10 drops of
triethylamine.
Question: Does triethylamine dissolve in water?
B-3 Then add 15 drops of conc. HCl to the above beaker. Stir and check the pH of solution
using blue litmus paper.
Question: Does it dissolve when conc. HCl is added?
Question: Complete the following chemical reaction.
HCI
C. Hydrolysis of Amides (Demonstration)
Place a few crystals of acetamide in a clean test tube.
Add approximately 2mL of 6M NaOH.
Warm gently in a boiling water bath.
Carefully note the odor of gas evolved.
Hold a piece of moist red litmus paper above the heated test tube.
Question: Identify the gas evolved.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

