Using your knowledge of the Brønsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) b) HNO3 + OH-→ CH3NH2 + H20 → OH- + HPO4-2 →
Using your knowledge of the Brønsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) b) HNO3 + OH-→ CH3NH2 + H20 → OH- + HPO4-2 →
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.17E: Write equations to illustrate the acid-base reaction when each of the following pairs of Brnsted...
Related questions
Question
100%
Hello. Could you please answer this question but please use the Ka values of each compound so that I can learn how to identify which is my acid and which is my base.
I am really struggling with 1B because I don't understand why water is serving as an acid when its Ka value is smaller than the Ka value of CH3NH2
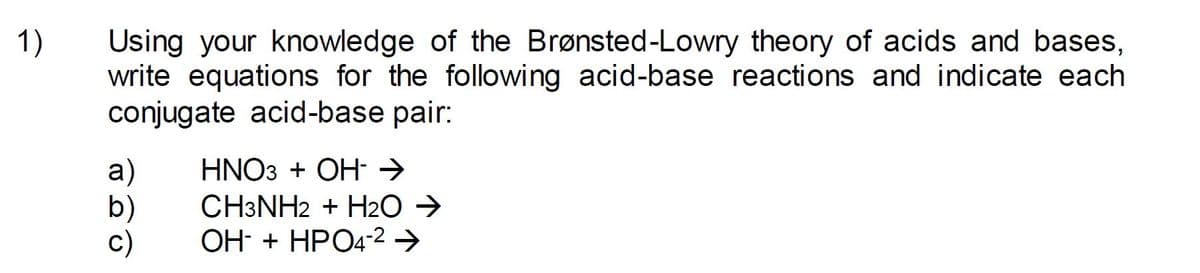
Transcribed Image Text:1)
Using your knowledge of the Brønsted-Lowry theory of acids and bases,
write equations for the following acid-base reactions and indicate each
conjugate acid-base pair:
HNO3 + OH- →
а)
b)
c)
CH3NH2 + H2O →
OH- + HPO4-2 →
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning