A. Calculate the concentration of sugar in this solution using the volume percent equation. For the purpose of this exercise, it can be assumed that the weight of the drink mix is 0.00 g. The volume of the solution is 3 and 1/3 cups. Calculate and record the volume percent (g/mL). (Note that 1 cup = 236 mL). Volume %=Volume of Solute / Volume of Solution ×100% I used 1/2 cup of granulated sugar and 1/2 tsp of the drink mix, and 3 cups of water. B. Calculate the concentration of each diluted solution using the equation below and the volumes in Table. C1V1=C2V2 Where: C1=concentration of stock solution V1=volume of stock solution C2=concentration of diluted solution V2=volume of diluted solution (This is all the information that I have)
A. Calculate the concentration of sugar in this solution using the volume percent equation. For the purpose of this exercise, it can be assumed that the weight of the drink mix is 0.00 g. The volume of the solution is 3 and 1/3 cups. Calculate and record the volume percent (g/mL). (Note that 1 cup = 236 mL).
Volume %=Volume of Solute / Volume of Solution ×100%
I used 1/2 cup of granulated sugar and 1/2 tsp of the drink mix, and 3 cups of water.
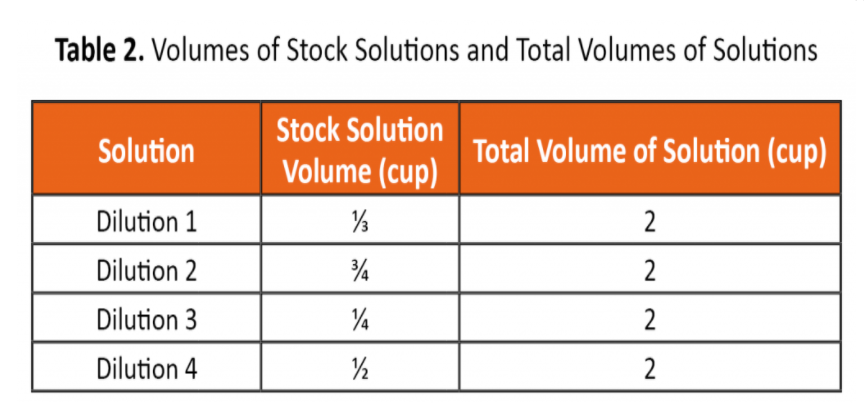
B. Calculate the concentration of each diluted solution using the equation below and the volumes in Table.
C1V1=C2V2
Where:
C1=concentration of stock solution
V1=volume of stock solution
C2=concentration of diluted solution
V2=volume of diluted solution
(This is all the information that I have)
Trending now
This is a popular solution!
Step by step
Solved in 3 steps









