A. H C. 23892U Label each of the following as stable or unstable. Briefly describe your reasoning. C. 210 PO 2002Pb + D. Balance the following nuclear reactions by filling in the blank with the missing product. A. Thorium (20 Th) gives of an alpha particle and becomes radium (Ra). B. 214Pb 214B + E. →214Pb + He B. H 234Pa + e D. Na
A. H C. 23892U Label each of the following as stable or unstable. Briefly describe your reasoning. C. 210 PO 2002Pb + D. Balance the following nuclear reactions by filling in the blank with the missing product. A. Thorium (20 Th) gives of an alpha particle and becomes radium (Ra). B. 214Pb 214B + E. →214Pb + He B. H 234Pa + e D. Na
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter21: Nuclear Chemistry
Section: Chapter Questions
Problem 30E: Write a nuclear reaction for each step in the formation of P82208b from T90228h, which proceeds by a...
Related questions
Question
100%
Give a clear handwritten answer with explanation..please give answer sub parts
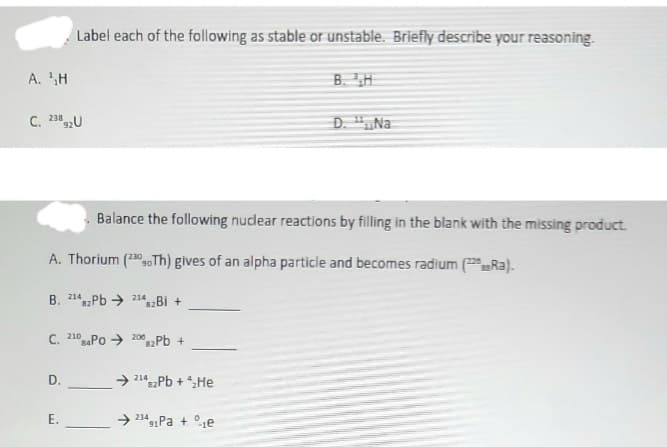
Transcribed Image Text:A. H
C. 23892U
Label each of the following as stable or unstable. Briefly describe your reasoning.
C. 210 PO 2002Pb +
D.
Balance the following nuclear reactions by filling in the blank with the missing product.
A. Thorium (230 Th) gives of an alpha particle and becomes radium (Ra).
B. 2142Pb 214B +
82
E.
→2142Pb + ₂He
82
B. H
➜ 234 Pa + e
91
D. "Na
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning