A. phosphorous (II) oxide B. potassium (V) oxide C. potassium (II) oxide D. phosphorous (V) oxide A comparison of calcium sulfate and calcium sulfite shows what? A. both have a monatomic cation and a polyatomic ion B. calcium sulfite has more oxygen atoms than calcium sulfate C. only calcium sulfite contains a polyatomic ion D. only calcium sulfate is arranged in a crystal lattice pattern 07. Balance the following equation,, What is the sum of all the coefficients in the balanced equation? 08. C2H6 + O2 CO2 H2. С. 19 D. 24 А. 14 В. 17 What is similar to these two salts NaCl and CaCl, ? A. are good conductors of electricity B. have the same crystal lattice structure 09. C. have positive charges D. are held together by ionic bond
A. phosphorous (II) oxide B. potassium (V) oxide C. potassium (II) oxide D. phosphorous (V) oxide A comparison of calcium sulfate and calcium sulfite shows what? A. both have a monatomic cation and a polyatomic ion B. calcium sulfite has more oxygen atoms than calcium sulfate C. only calcium sulfite contains a polyatomic ion D. only calcium sulfate is arranged in a crystal lattice pattern 07. Balance the following equation,, What is the sum of all the coefficients in the balanced equation? 08. C2H6 + O2 CO2 H2. С. 19 D. 24 А. 14 В. 17 What is similar to these two salts NaCl and CaCl, ? A. are good conductors of electricity B. have the same crystal lattice structure 09. C. have positive charges D. are held together by ionic bond
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 117QRT
Related questions
Question
please answer all
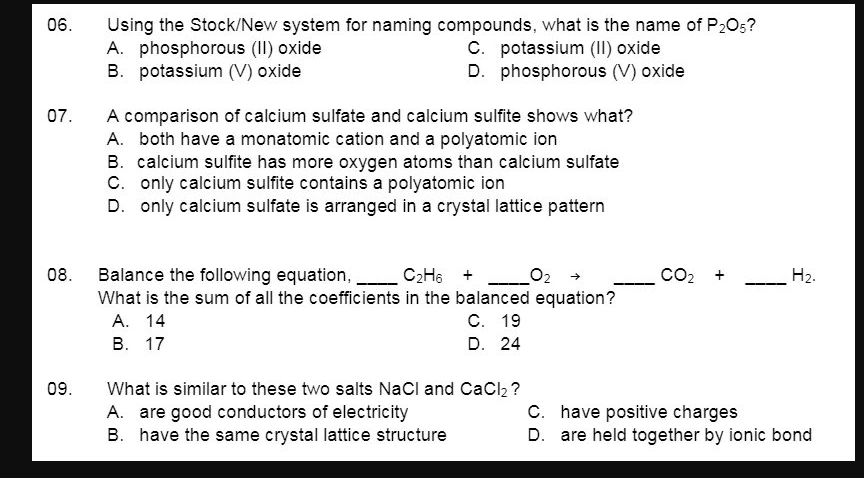
Transcribed Image Text:06.
Using the Stock/New system for naming compounds, what is the name of P205?
A. phosphorous (II) oxide
B. potassium (V) oxide
C. potassium (II) oxide
D. phosphorous (V) oxide
07.
A comparison of calcium sulfate and calcium sulfite shows what?
A. both have a monatomic cation and a polyatomic ion
B. calcium sulfite has more oxygen atoms than calcium sulfate
C. only calcium sulfite contains a polyatomic ion
D. only calcium sulfate is arranged in a crystal lattice pattern
CO2 +
Balance the following equation,
What is the sum of all the coefficients in the balanced equation?
А. 14
В. 17
08.
C2H6 + O:
H2.
С. 19
D. 24
What is similar to these two salts NaCl and CaCl, ?
A. are good conductors of electricity
B. have the same crystal lattice structure
09.
C. have positive charges
D. are held together by ionic bond
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
