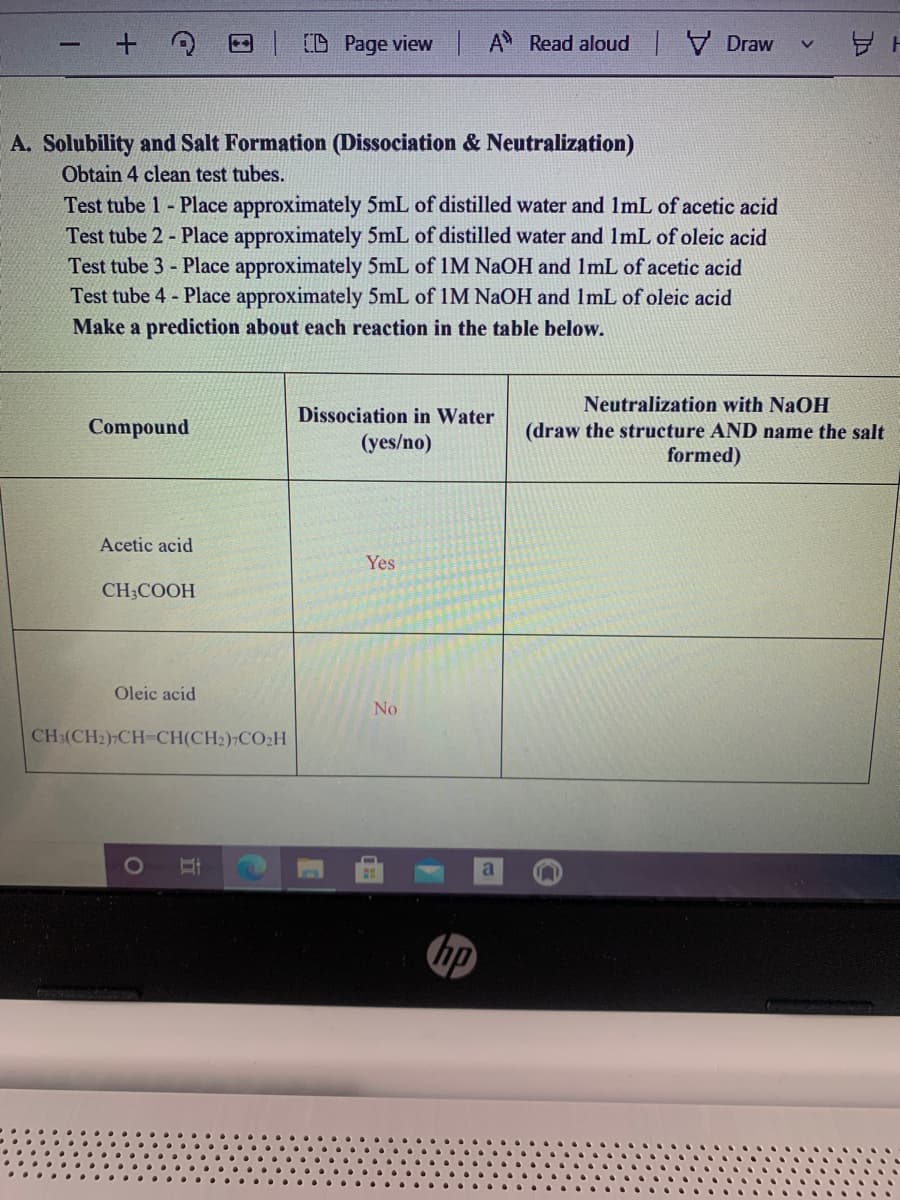
A. Solubility and Salt Formation (Dissociation & Neutralization) Obtain 4 clean test tubes. Test tube 1 - Place approximately 5mL of distilled water and 1mL of acetic acid Test tube 2 - Place approximately 5mL of distilled water and 1mL of oleic acid Test tube 3 - Place approximately 5mL of IM NaOH and ImL of acetic acid Test tube 4 - Place approximately 5mL of IM NAOH and ImL of oleic acid Make a prediction about each reaction in the table below. Neutralization with NaOH (draw the structure AND name the sa. formed) Dissociation in Water Compound (yes/no)
A. Solubility and Salt Formation (Dissociation & Neutralization) Obtain 4 clean test tubes. Test tube 1 - Place approximately 5mL of distilled water and 1mL of acetic acid Test tube 2 - Place approximately 5mL of distilled water and 1mL of oleic acid Test tube 3 - Place approximately 5mL of IM NaOH and ImL of acetic acid Test tube 4 - Place approximately 5mL of IM NAOH and ImL of oleic acid Make a prediction about each reaction in the table below. Neutralization with NaOH (draw the structure AND name the sa. formed) Dissociation in Water Compound (yes/no)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter6: Solutions And Colloids
Section: Chapter Questions
Problem 6.71P: 6-71 A 4 M acetic acid (CH3COOH) solution lowers the freezing point by-8°C; a 4 M KF solution yields...
Related questions
Question
Transcribed Image Text:(D Page view A Read aloud V Draw
A. Solubility and Salt Formation (Dissociation & Neutralization)
Obtain 4 clean test tubes.
Test tube 1 - Place approximately 5mL of distilled water and 1mL of acetic acid
Test tube 2 - Place approximately 5mL of distilled water and ImL of oleic acid
Test tube 3 - Place approximately 5mL of IM NaOH and ImL of acetic acid
Test tube 4 - Place approximately 5mL of IM NAOH and ImL of oleic acid
Make a prediction about each reaction in the table below.
Neutralization with NaOH
Dissociation in Water
Compound
(draw the structure AND name the salt
formed)
(yes/no)
Acetic acid
Yes
CH;COOH
Oleic acid
No
CH:(CH2)-CH-CH(CH2) CO2H
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning