An ionic solid consists of A atoms and B atoms, and its unit cell structure is shown below. The position of A atoms (orange color) and the position of B atoms (blue color) in the cubic unit cell are indicated in the picture. Based on the cubic unit cell given here, which statement is correct? A= edge B=comer, center O a. The empirical formula of this ionic compound is A12B5. O b. The empirical formula of this ionic compound is A3B2. O. The empirical formula of this ionic compound is A2B3. d. The empirical formula of this ionic compound is AB3. e. None of the above
An ionic solid consists of A atoms and B atoms, and its unit cell structure is shown below. The position of A atoms (orange color) and the position of B atoms (blue color) in the cubic unit cell are indicated in the picture. Based on the cubic unit cell given here, which statement is correct? A= edge B=comer, center O a. The empirical formula of this ionic compound is A12B5. O b. The empirical formula of this ionic compound is A3B2. O. The empirical formula of this ionic compound is A2B3. d. The empirical formula of this ionic compound is AB3. e. None of the above
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section: Chapter Questions
Problem 29PS: A diamond unit cell is shown here. Unit cell of diamond (a) How many carbon atoms are in one unit...
Related questions
Question
Thank you for your help!
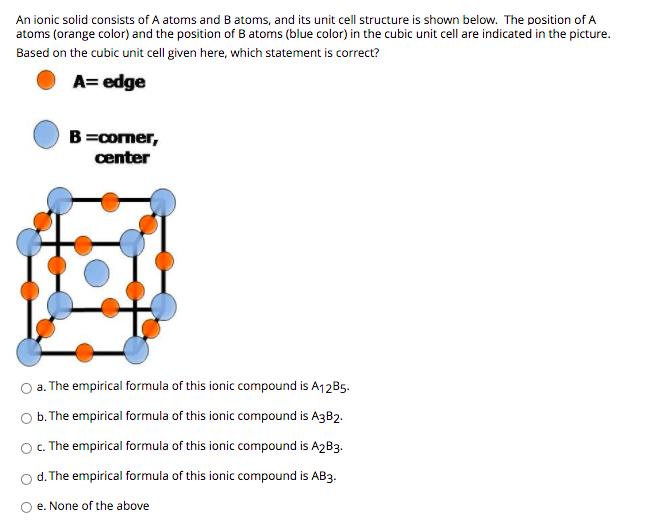
Transcribed Image Text:An ionic solid consists of A atoms and Batoms, and its unit cell structure is shown below. The position of A
atoms (orange color) and the position of B atoms (blue color) in the cubic unit cell are indicated in the picture.
Based on the cubic unit cell given here, which statement is correct?
A= edge
B=comer,
center
O a. The empirical formula of this ionic compound is A12B5.
O . The empirical formula of this ionic compound is A3B2.
O C. The empirical formula of this ionic compound is A2B3.
d. The empirical formula of this ionic compound is AB3.
O e. None of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning